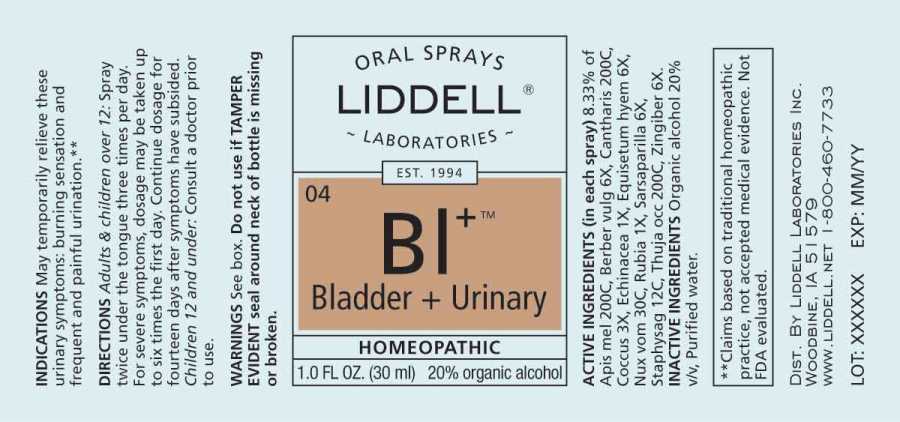
ACTIVE INGREDIENTS:
(in each drop): 8.33% of Apis Mellifica 200C, Berberis Vulgaris 6X, Cantharis 200C, Coccus Cacti 3X, Echinacea (Angustifolia) 1X, Equisetum Hyemale 6X, Nux Vomica 30C, Rubia Tinctorum 1X, Sarsaparilla (Smilax Regelii) 6X, Staphysagria 12C, Thuja Occidentalis 200C, Zingiber Officinale 6X.
USES:
May temporarily relieve these urinary symptoms:
- burning sensation
- frequent need to urinate
- painful urination**
**Claims based on traditional homeopathic practice, not accepted medical evidence. Not FDA evaluated.
WARNINGS:
Do not use if you have ever has an allergic reaction to this product or any of its ingredients.
Stop use and ask a doctor if symptoms persist for more than 3 days, are severe, or you experience fever, chills, back pain or bloody urine.
You may have a serious condition that requires professional treatment.
Keep out of reach of children. In case of overdose, get medical help or call a Poison Control Center right away.
If pregnant or breast-feeding, ask a health professional before use.
Do not use if TAMPER EVIDENT seal around neck of bottle is missing or broken.
KEEP OUT OF REACH OF CHILDREN:
Keep out of reach of children. In case of overdose, get medical help or call a Poison Control Center right away.
DIRECTIONS:
Adults and children over 12: Spray twice under the tongue three times per day.
For severe symptoms, dosage may be taken up to six times the first day. Continue dosage for fourteen days after symptoms have subsided.
Children 12 and under: Consult a doctor prior to use.
INDICATIONS:
May temporarily relieve these urinary symptoms:
• burning sensation
• frequent need to urinate
• painful urination**
**Claims based on traditional homeopathic practice, not accepted medical evidence. Not FDA evaluated.