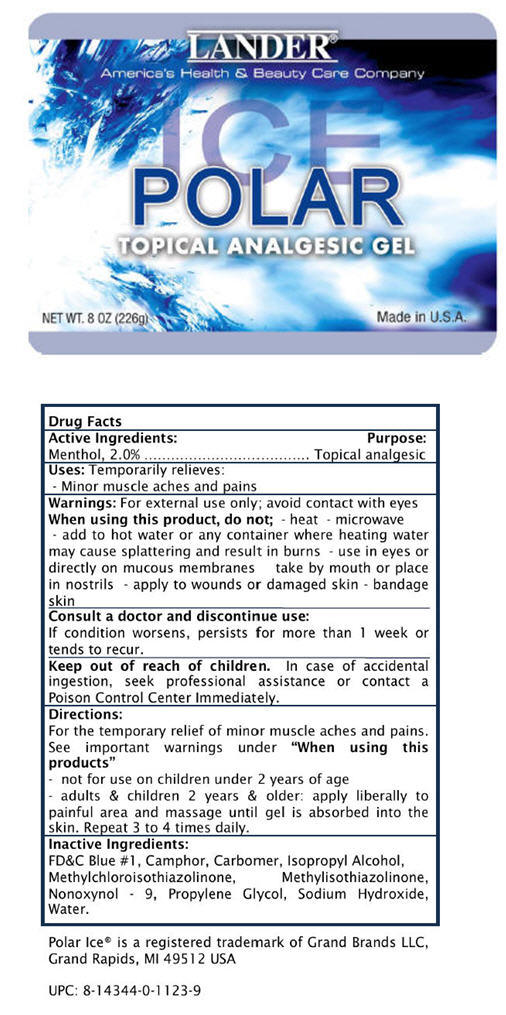
LANDER POLAR ICE- menthol gel
Abaco Partners LLC DBA Surefil
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active Ingredients
Menthol, 2.0%
Purpose
Topical analgesic
Uses
Temporarily relieves:
- -
- Minor muscle aches and pains
Warnings
For external use only; avoid contact with eyes
When using this product, do not;
- -
- heat
- -
- microwave
- -
- add to hot water or any container where heating water may cause splattering and result in burns
- -
- use in eyes or directly on mucous membranes
-
- take by mouth or place in nostrils
- -
- apply to wounds or damaged skin
- -
- bandage skin
Consult a doctor and discontinue use:
If condition worsens, persists for more than 1 week or tends to recur.
Keep out of reach of children. In case of accidental ingestion, seek professional assistance or contact a Poison Control Center Immediately.
Directions
For the temporary relief of minor muscle aches and pains. See important warnings under "When using this products"
- -
- not for use on children under 2 years of age
- -
- adults & children 2 years & older: apply liberally to painful area and massage until gel is absorbed into the skin. Repeat 3 to 4 times daily.
Inactive Ingredients
FD&C Blue #1, Camphor, Carbomer, Isopropyl Alcohol, Methylchloroisothiazolinone, Methylisothiazolinone, Nonoxynol - 9, Propylene Glycol, Sodium Hydroxide, Water.
Polar Ice® is a registered trademark of Grand Brands LLC, Grand
Rapids, MI 49512 USA
UPC: 8-14344-0-1123-9
PRINCIPAL DISPLAY PANEL - 226g Container
LANDER®
America's Health & Beauty Care Company
POLAR
ICE
TOPICAL ANALGESIC GEL
NET WT. 8 OZ (226g)
Made in U.S.A.