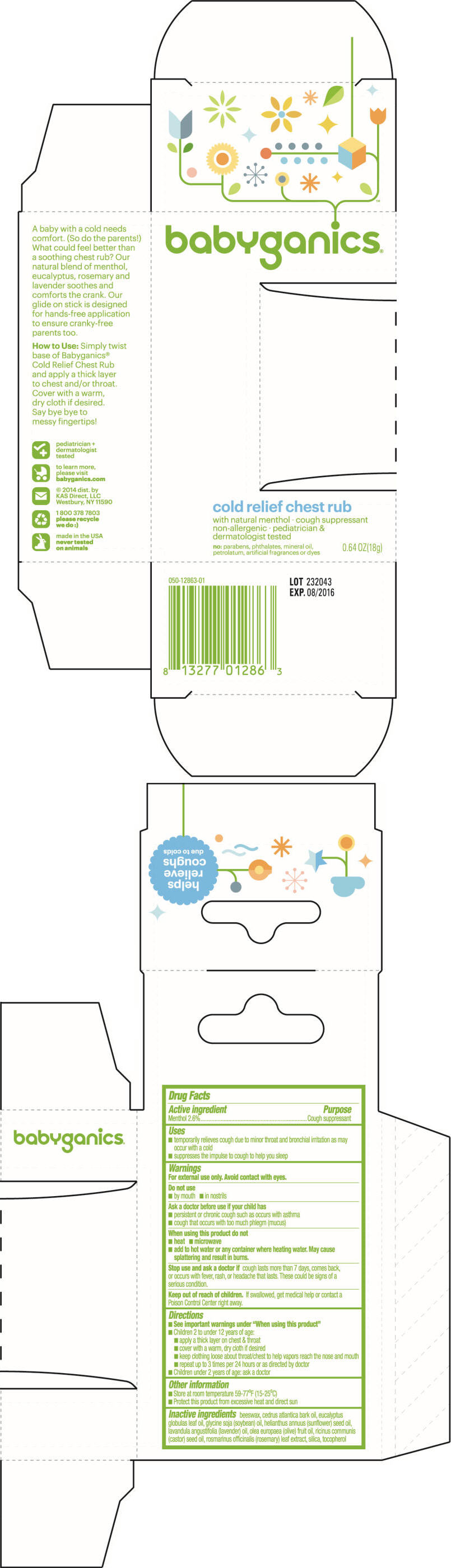
Uses
- temporarily relieves cough due to minor throat and bronchial irritation as may occur with a cold
- suppresses the impulse to cough to help you sleep
Warnings
For external use only. Avoid contact with eyes.
Ask a doctor before use if your child has
- persistent or chronic cough such as occurs with asthma
- cough that occurs with too much phlegm (mucus)
When using this product do not
- heat
- microwave
- add to hot water or any container where heating water. May cause splattering and result in burns.
Directions
- see important warnings under "When using this product"
- children 2 to under 12 years of age:
- apply a thick layer on chest & throat
- cover with a warm, dry cloth if desired
- keep clothing loose about throat/chest to help vapors reach the nose and mouth
- repeat up to 3 times per 24 hours or as directed by doctor
- children under 2 years of age: ask a doctor
Other information
- store at room temperature 59-77°F (15-25°C)
- protect this product from excessive heat and direct sun
Inactive ingredients
beeswax, cedrus atlantica bark oil, eucalyptus globulas leaf oil, glycine soja (soybean) oil, helianthus annuus (sunflower) seed oil, lavandula angustifolia (lavender) oil, olea europaea (olive) fruit oil, ricinus communis (castor) seed oil, rosmarinus officinalis (rosemary) leaf extract, silica, tocopherol