DESCRIPTION
The active ingredient in CANASA® 1000 mg suppositories is mesalamine, also known as mesalazine or 5-aminosalicylic acid (5-ASA). Chemically, mesalamine is 5-amino-2-hydroxybenzoic acid, and is classified as an anti-inflammatory drug.
The empirical formula is C7H7NO3, representing a molecular weight of 153.14. The structural formula is:
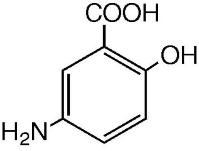
Each CANASA® rectal suppository contains 1000 mg of mesalamine (USP) in a base of Hard Fat, NF.
CLINICAL PHARMACOLOGY
Sulfasalazine has been used in the treatment of ulcerative colitis for over 55 years. It is split by bacterial action in the colon into sulfapyridine (SP) and mesalamine (5-ASA). It is thought that the mesalamine component only is therapeutically active in ulcerative colitis.
Mechanism of ActionThe mechanism of action of mesalamine (and sulfasalazine) is not fully understood, but appears to be topical rather than systemic. Although the pathology of inflammatory bowel disease is uncertain, both prostaglandins and leukotrienes have been implicated as mediators of mucosal injury and inflammation. Recently, however, the role of mesalamine as a free radical scavenger or inhibitor of tumor necrosis factor (TNF) has also been postulated.
PharmacokineticsAbsorption:Mesalamine (5-ASA) administered as a rectal suppository is variably absorbed. In patients with ulcerative colitis treated with mesalamine 500 mg rectal suppositories, administered once every eight hours for six days, the mean mesalamine peak plasma concentration (Cmax) was 353 ng/mL (CV=55%) following the initial dose and 361 ng/mL (CV=67%) at steady state. The mean minimum steady state plasma concentration (Cmin) was 89 ng/mL (CV=89%). Absorbed mesalamine does not accumulate in the plasma.
Distribution:Mesalamine administered as rectal suppositories distributes in rectal tissue to some extent. In patients with ulcerative proctitis treated with CANASA® (mesalamine, USP) 1000 mg rectal suppositories, rectal tissue concentrations for 5-ASA and N-acetyl-5-ASA have not been rigorously quantified.
Metabolism:Mesalamine is extensively metabolized, mainly to N-acetyl-5-ASA. The site of metabolism has not been elucidated. In patients with ulcerative colitis treated with one 500 mg mesalamine rectal suppository every eight hours for six days, peak concentration (Cmax) of N-acetyl-5-ASA ranged from 467 ng/mL to 1399 ng/mL following the initial dose and from 193 ng/mL to 1304 ng/mL at steady state.
Elimination:Mesalamine is eliminated from plasma mainly by urinary excretion, predominantly as N-acetyl-5-ASA. In patients with ulcerative proctitis treated with one mesalamine 500 mg rectal suppository every eight hours for six days, ≤ 12% of the dose was eliminated in urine as unchanged 5-ASA and 8-77% as N-acetyl-5-ASA following the initial dose. At steady state, ≤ 11% of the dose was eliminated as unchanged 5-ASA and 3-35% as N-acetyl-5-ASA. The mean elimination half-life was five hours (CV=73%) for 5-ASA and six hours (CV=63%) for N-acetyl-5-ASA following the initial dose. At steady state, the mean elimination half-life was seven hours for both 5-ASA and N-acetyl-5-ASA (CV=102% for 5-ASA and 82% for N-acetyl-5-ASA).
Drug-Drug Interactions:The potential for interactions between mesalamine, administered as 1000 mg rectal suppositories, and other drugs has not been studied.
Special Populations (Patients with Renal or Hepatic Impairment):The effect of renal or hepatic impairment on elimination of mesalamine in ulcerative proctitis patients treated with mesalamine 1000 mg suppositories has not been studied.
Preclinical ToxicologyPreclinical studies of mesalamine were conducted in rats, mice, rabbits and dogs, and kidney was the main target organ of toxicity. In rats, adverse renal effects were observed at a single oral dose of 600 mg/kg (about 3.2 times the recommended human intra-rectal dose, based on body surface area) and at IV doses of >214 mg/kg (about 1.2 times the recommended human intra-rectal dose, based on body surface area). In a 13-week oral gavage toxicity study in rats, papillary necrosis and/or multifocal tubular injury were observed in males receiving 160 mg/kg (about 0.86 times the recommended human intra-rectal dose, based on body surface area) and in both males and females at 640 mg/kg (about 3.5 times the recommended human intra-rectal dose, based on body surface area). In a combined 52-week toxicity and 127-week carcinogenicity study in rats, degeneration of the kidneys and hyalinization of basement membranes and Bowman’s capsule were observed at oral doses of 100 mg/kg/day (about 0.54 times the recommended human intra-rectal dose, based on body surface area) and above. In a 14-day rectal toxicity study of mesalamine suppositories in rabbits, intra-rectal doses up to 800 mg/kg (about 8.6 times the recommended human intra-rectal dose, based on body surface area) was not associated with any adverse effects. In a six-month oral toxicity study in dogs, doses of 80 mg/kg (about 1.4 times the recommended human intra-rectal dose, based on body surface area) and higher caused renal pathology similar to that described for the rat. In a rectal toxicity study of mesalamine suppositories in dogs, a dose of 166.6 mg/kg (about 3.0 times the recommended human intra-rectal dose, based on body surface area) produced chronic nephritis and pyelitis. In the 12-month eye toxicity study in dogs, Keratoconjunctivitis sicca (KCS) occurred at oral doses of 40 mg/kg (about 0.72 times the recommended human intra-rectal dose, based on body surface area) and above.
CLINICAL STUDIES
Two double-blind placebo-controlled multicenter studies were conducted in North America in patients with mild to moderate active ulcerative proctitis. The primary measures of efficacy were the same in all trials (clinical disease activity index, sigmoidoscopic and histologic evaluations). The main difference between the studies was dosage regimen: 500 mg three times daily (1.5 g/d) in Study 1; and 500 mg twice daily (1.0 g/d) in Study 2. A total of 173 patients were studied (Study 1, N=79; Study 2, N=94). Eighty-nine (89) patients received mesalamine suppositories, and eighty-four (84) patients received placebo suppositories. Patients were evaluated clinically and sigmoidoscopically after three and six weeks of suppository treatment. In Study No. 1 patients were 17 to 73 years of age (mean = 39 yrs), 57% were female, and 97% were white. Patients had an average extent of proctitis (upper disease boundary) of 10.8 cm. Eighty-four percent (84%) of the study patients had multiple prior episodes of proctitis. In Study No. 2, patients were 21 to 72 years of age (mean = 39 yrs), 62% were female, and 96% were white. Patients had an average extent of proctitis (upper disease boundary) of 10.3 cm. Seventy-eight percent (78%) of the study patients had multiple prior episodes of proctitis.
Compared to placebo, mesalamine suppository treatment was statistically (p less than 0.01) superior to placebo in all trials with respect to improvement in stool frequency, rectal bleeding, mucosal appearance, disease severity, and overall disease activity after three and six weeks of treatment. Daily diary records indicated significant improvement in rectal bleeding in the first week of therapy while tenesmus and diarrhea improved significantly within two weeks. Investigators rated patients receiving mesalamine much improved compared to patients receiving placebo (p less than 0.001).
The effectiveness of mesalamine suppositories was statistically significant irrespective of sex, extent of proctitis, duration of current episode or duration of disease.
A multicenter, open-label, randomized, parallel group study in ninety-nine (99) patients diagnosed with mild to moderate ulcerative proctitis compared the clinical efficacy of the CANASA® 1000 mg suppository to that of the CANASA ® 500 mg suppository. The primary measures of efficacy included clinical disease activity index, sigmoidoscopic and histologic evaluations. Patients were randomized to one of two treatment groups, with a dosage regimen of one 500 mg mesalamine suppository BID, morning and HS, or one 1000 mg mesalamine suppository HS for 6 weeks. Patients were evaluated clinically and sigmoidoscopically after three and six weeks of suppository treatment. Of the eighty-one (81) patients in the Per Protocol population, forty-six (46) patients received mesalamine 500 mg suppositories BID, and thirty-five (35) patients received mesalamine 1000 mg suppositories HS.
The efficacy of the 1000 mg HS treatment was not statistically or clinically different after 6 weeks from the 500 mg BID treatment, and both were effective in the treatment of ulcerative proctitis. Both treatments resulted in a significant decrease between Baseline and 6 weeks in the Disease Activity Index (DAI), a composite index reflecting rectal bleeding, stool frequency, mucosal appearance at endoscopy, and a global assessment of disease. In the 500 mg BID group, the mean DAI value decreased from 6.6 to 1.6, and in the 1000 mg HS group, the mean DAI value decreased from 6.2 to 1.3 over 6 weeks of treatment, representing a decrease of greater than 75% in both groups. Seventy-eight percent (78%; 36/46) of patients in the 500 mg BID group and 86% (30/35) of the patients in the 1000 mg HS group achieved a substantial improvement in symptoms (defined as a DAI score of less than 3) after 6 weeks of treatment. These patients regained normal daily stools, lost their rectal bleeding, and lost signs of inflammation at endoscopic visualization. The time to onset of response to the study drug was within 3 weeks of initiation of therapy in each treatment group, but further improvement was observed between 3 and 6 weeks of treatment.
INDICATIONS AND USAGE
CANASA® 1000 mg Suppositories are indicated for the treatment of active ulcerative proctitis.
CONTRAINDICATIONS
CANASA® 1000 mg Suppositories are contraindicated in patients who have demonstrated hypersensitivity to mesalamine (5-aminosalicylic acid) or to the suppository vehicle [saturated vegetable fatty acid esters (Hard Fat, NF)], or to salicylates (including aspirin).
PRECAUTIONS
Mesalamine has been implicated in the production of an acute intolerance syndrome characterized by cramping, acute abdominal pain and bloody diarrhea, sometimes fever, headache and a rash; in such cases prompt withdrawal is required. The patient’s history of sulfasalazine intolerance, if any, should be re-evaluated. If a rechallenge is performed later in order to validate the hypersensitivity, it should be carried out under close supervision and only if clearly needed, giving consideration to reduced dosage. In the literature, one patient previously sensitive to sulfasalazine was rechallenged with 400 mg oral mesalamine; within eight hours she experienced headache, fever, intensive abdominal colic, profuse diarrhea and was readmitted as an emergency. She responded poorly to steroid therapy and two weeks later a pancolectomy was required. The possibility of increased absorption of mesalamine and concomitant renal tubular damage as noted in the preclinical studies must be kept in mind. Patients on CANASA® 1000 mg, especially those on concurrent oral products which contain or release mesalamine and those with pre-existing renal disease, should be carefully monitored with urinalysis, BUN and creatinine testing.
In a clinical trial most patients who were hypersensitive to sulfasalazine were able to take mesalamine enemas without evidence of any allergic reaction. Nevertheless, caution should be exercised when mesalamine is initially used in patients known to be allergic to sulfasalazine. These patients should be instructed to discontinue therapy if signs of rash or fever become apparent.
A small proportion of patients have developed pancolitis while using mesalamine. However, extension of upper disease boundary and/or flare-ups occurred less often in the mesalamine-treated group than in the placebo-treated group.
Rare instances of pericarditis have been reported with mesalamine containing products including sulfasalazine. Cases of pericarditis have also been reported as manifestations of inflammatory bowel disease. In the cases reported there have been positive rechallenges with mesalamine or mesalamine containing products. In one of these cases, however, a second rechallenge with sulfasalazine was negative throughout a 2 month follow-up. Chest pain or dyspnea in patients treated with mesalamine should be investigated with this information in mind. Discontinuation of CANASA® suppositories may be warranted in some cases, but rechallenge with mesalamine can be performed under careful clinical observation should the continued therapeutic need for mesalamine be present.
There have been two reports in the literature of additional serious adverse events: one patient who developed leukopenia and thrombocytopenia after seven months of treatment with one 500 mg suppository nightly, and one patient with rash and fever which was a similar reaction to sulfasalazine.
Information for Patients:See patient information printed at the end of this insert.
Carcinogenesis, Mutagenesis, Impairment of FertilityMesalamine caused no increase in the incidence of neoplastic lesions over controls in a two-year study of Wistar rats fed up to 320 mg/kg/day of mesalamine admixed with diet (about 1.7 times the recommended human intra-rectal dose, based on body surface area).
Mesalamine was not mutagenic in the Ames test, the mouse lymphoma cell (TK±) forward mutation test, or the mouse micronucleus test.
No effects on fertility or reproductive performance of the male and female rats were observed at oral mesalamine doses up to 320 mg/kg/day (about 1.7 times the recommended human intra-rectal dose, based on body surface area). The oligospermia and infertility in men associated with sulfasalazine have not been reported with mesalamine.
PregnancyTeratogenic Effects, Pregnancy Category BTeratology studies have been performed in rats at oral doses up to 320 mg/kg/day (about 1.7 times the recommended human intra-rectal dose, based on body surface area) and in rabbits at oral doses up to 495 mg/kg/day (about 5.4 times the recommended human intra-rectal dose, based on body surface area) and have revealed no evidence of impaired fertility or harm to the fetus due to mesalamine. There are, however, no adequate and well controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used in pregnancy only if clearly needed.
Nursing MothersIt is not known whether mesalamine or its metabolite(s) are excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when CANASA® 1000 mg suppositories are administered to a nursing woman.
Pediatric UseSafety and effectiveness in pediatric patients have not been established.
Geriatric UseClinical studies of CANASA® did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Mesalamine is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, it may be useful to monitor renal function.
ADVERSE REACTIONS
Clinical Adverse ExperienceThe most frequent adverse reactions observed in the double-blind, placebo-controlled trials are summarized in the Table below.
| Symptom | Mesalamine
(n=177) | Placebo
(n=84) |
||
| N | % | N | % | |
| Dizziness | 5 | 3.0 | 2 | 2.4 |
| Rectal Pain | 3 | 1.8 | 0 | 0.0 |
| Fever | 2 | 1.2 | 0 | 0.0 |
| Rash | 2 | 1.2 | 0 | 0.0 |
| Acne | 2 | 1.2 | 0 | 0.0 |
| Colitis | 2 | 1.2 | 0 | 0.0 |
In the multicenter, open-label, randomized, parallel group study comparing the CANASA® 1000 mg suppository (HS) to that of the CANASA® 500 mg suppository (BID), there were no differences between the two treatment groups in the adverse event profile. The most frequent AEs were headache (14.4%), flatulence (5.2%), abdominal pain (5.2%), diarrhea (3.1%), and nausea (3.1%). Three (3) patients had to discontinue medication because of a treatment emergent AE; one of these AEs (headache) was deemed possibly related to study medication.
In addition to the events observed in the clinical trials, the following adverse events have been associated with mesalamine containing products: nephrotoxicity, pancreatitis, fibrosing alveolitis and elevated liver enzymes. Cases of pancreatitis and fibrosing alveolitis have been reported as manifestations of inflammatory bowel disease as well.
Hair LossMild hair loss characterized by “more hair in the comb” but no withdrawal from clinical trials has been observed in seven of 815 mesalamine patients but none of the placebo-treated patients. In the literature there are at least six additional patients with mild hair loss who received either mesalamine or sulfasalazine. Retreatment is not always associated with repeated hair loss.
OVERDOSAGE
There have been no documented reports of serious toxicity in man resulting from massive overdosing with mesalamine. Under ordinary circumstances, mesalamine absorption from the colon is limited.
DOSAGE AND ADMINISTRATION
The usual dosage of CANASA® (mesalamine, USP) 1000 mg suppositories is one rectal suppository 1 time daily at bedtime.
The suppository should be retained for one to three hours or longer, if possible, to achieve the maximum benefit. While the effect of CANASA® suppositories may be seen within three to twenty-one days, the usual course of therapy would be from three to six weeks depending on symptoms and sigmoidoscopic findings. Studies have suggested that CANASA® suppositories will delay relapse after the six-week short-term treatment.
Patient Instructions:NOTE: CANASA® suppositories will cause staining of direct contact surfaces, including but not limited to fabrics, flooring, painted surfaces, marble, granite, vinyl, and enamel.
- Detach one suppository from strip of suppositories.
- Hold suppository upright and carefully remove the plastic wrapper.
- Avoid excessive handling of suppository, which is designed to melt at body temperature.
- Insert suppository completely into rectum with gentle pressure, pointed end first.
- A small amount of lubricating gel may be used on the tip of the suppository to assist insertion.
HOW SUPPLIED
CANASA® (Mesalamine, USP) 1000 mg Suppositories: CANASA® 1000 mg Suppositories for rectal administration are available as bullet shaped, light tan suppositories containing 1000 mg mesalamine supplied in boxes of 30 plastic wrapped suppositories (NDC 54868-6147-0).
Store below 25°C (77°F), do not freeze. Keep away from direct heat, light or humidity.
Rx only
Axcan Pharma US, Inc.
Birmingham, AL 35242
Date: May 2008
Relabeling of "Additional Barcode Label" by:
Physicians Total Care, Inc.
Tulsa, OK 74146
CANASA® Rectal Suppositories
(Mesalamine, USP) 1000 mg
Read this information carefully before you begin treatment. Also, read the information you get whenever you get more medicine. There may be new information. This information does not take the place of talking with your doctor about your medical condition or your treatment. If you have any questions about this medicine, ask your doctor or pharmacist.
What is CANASA®?CANASA® (can-AH-sah) is a medicine used to treat ulcerative proctitis (ulcerative rectal colitis). CANASA® works inside your rectum (lower intestine) to help reduce bleeding, mucous and bloody diarrhea caused by inflammation (swelling and soreness) of the rectal area. You use CANASA® by inserting it into your rectum.
Who should not use CANASA®?Do not use CANASA® if you are allergic to the active ingredient mesalamine (also found in drugs such as Rowasa, Asacol, Pentasa, Azulfidine, and Dipentum), if you are allergic to the inactive ingredients, or if you have had any unusual reaction to the ingredients.
Tell your doctor if you:
- Have kidney problems. Using CANASA® may make them worse.
- Have had inflamed pancreas (pancreatitis).
- Are pregnant. You and your doctor will decide if you should use CANASA®.
- Have ever had pericarditis (inflamed sac around your heart).
- Are allergic to sulfasalazine. You may need to watch for signs of an allergic reaction to CANASA®.
- Are allergic to aspirin.
- Are allergic to other things, such as foods, preservatives, or dyes.
Follow your doctor’s instructions about how often to use CANASA® and how long to use it. For the 1000 mg suppository, the usual dose is one suppository at bedtime for 3-6 weeks. We do not know if CANASA® will work for children or is safe for them.
Follow these steps to use CANASA®:
- For best results, empty your rectum (have a bowel movement) just before using CANASA®.
- Detach one CANASA® suppository from the strip of suppositories.
- Hold the suppository upright and carefully peel open the plastic at the pre-cut line to take out the suppository.
- Insert the suppository with the pointed end first completely into your rectum, using gentle pressure.
- For best results, keep the suppository in your rectum for 3 hours or longer, if possible.
If you have trouble inserting CANASA®, you may put a little bit of lubricating gel on the suppository.
Do not handle the suppository too much, since it may begin to melt from the heat from your hands and body.
If you miss a dose of CANASA®, use it as soon as possible, unless it is almost time for next dose. Do not use two CANASA® suppositories at the same time to make up for a missed dose.
Keep using CANASA® as long as your doctor tells you to use it, even if you feel better.
CANASA® can cause stains on things it touches. Therefore keep it away from clothing and other fabrics, flooring, painted surfaces, marble, granite, plastics, andenamel. Be careful since CANASA® may stain clothing.
What should I avoid while taking CANASA®?Do not breast feed while using CANASA®. We do not know if CANASA® can pass through the milk and harm the baby. Tell your doctor if you become pregnant while using CANASA®.
What are the possible side effects of CANASA®?- The most common side effects of CANASA® are: headache, gas or flatulence, and diarrhea. These events also occurred when patients were given an inactive suppository.
- Less common, but possibly serious side effects include a reaction to the medicine (acute intolerance syndrome) that includes cramps, sharp abdominal (stomach area) pain, bloody diarrhea, and sometimes fever, headache and rash. Stop use and tell your doctor right away if you get any of these symptoms.
- In rare cases, the sac around the heart may become inflamed (pericarditis). Tell your doctor right away if you develop chest pain or shortness of breath, which are signs of this problem.
- In rare cases, patients using CANASA® develop worsening colitis (pancolitis).
- A very few patients using CANASA® may have mild hair loss.
- Other side effects not listed above may also occur in some patients.
If you notice any other side effects, check with your doctor or pharmacist.
How should I store CANASA®?Store CANASA® below 25°C (77°F), do not freeze it. Keep it away from direct heat, light, or humidity. Keep it out of the reach of children.
General advice about prescription medicinesMedicines are sometimes prescribed for conditions that are not mentioned in patient information leaflets. Do not use CANASA® for a condition for which it was not prescribed. Do not give CANASA® to other people, even if they have the same symptoms you have.
This leaflet summarizes the most important information about CANASA®. If you would like more information, talk with your doctor. You can ask your pharmacist or doctor for information about CANASA® that is written for health professionals.
