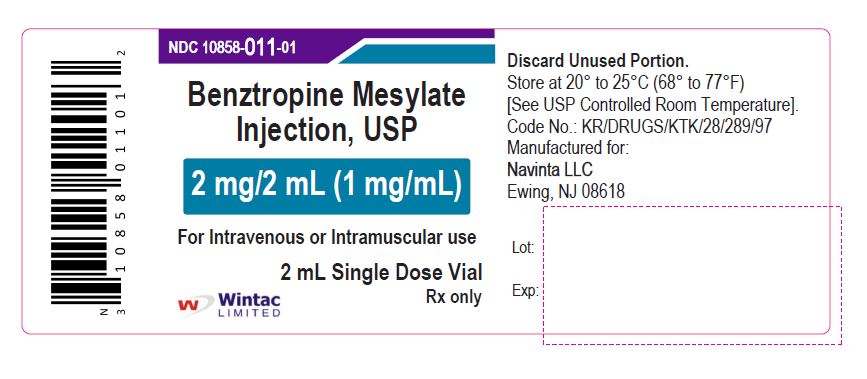
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Benztropine Mesylate
Injection, USP
2 mg/2 mL (1 mg/mL)
For Intravenous or Intramuscular use
2 mL Single Dose Vial
Wintac Limited
Rx only
vial
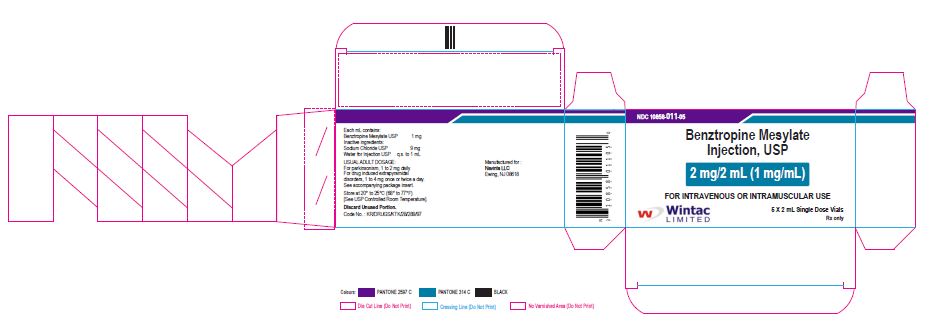
Benztropine Mesylate
Injection, USP
2 mg/2 mL (1 mg/mL)
FOR INTRAVENOUS OR INTRAMUSCULAR USE
Wintac Limited
5 X 2 mL Single Dose Vials
Rx only
carton