PACKAGE LABEL - PRINCIPAL DISPLAY PANEL
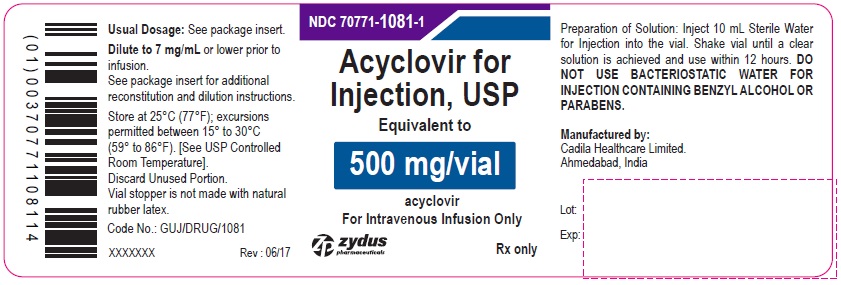
Acyclovir for Injection, USP 500 mg/vial - Vial Label
NDC 70771-1081-1
Acyclovir for Injection, USP
Equivalent to
500 mg/vial
acyclovir
For Intravenous Infusion Only
Rx only
zydus pharmaceuticals
Acyclovir for Injection, USP 500 mg/vial - Carton Label
NDC 70771-1081-6
Acyclovir for Injection, USP
Equivalent to
500 mg/vial
acyclovir
For Intravenous Infusion Only
10 x 500 mg vials
Rx only
zydus pharmaceuticals

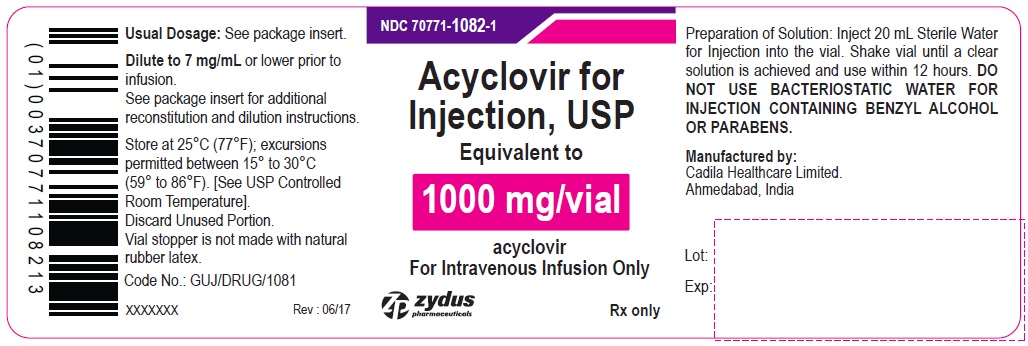
Acyclovir for Injection, USP 1000 mg/vial - Vial Label
NDC 70771-1082-1
Acyclovir for Injection, USP
Equivalent to
1000 mg/vial
acyclovir
For Intravenous Infusion Only
Rx only
zydus pharmaceuticals
Acyclovir for Injection, USP 1000 mg/vial - Carton Label
NDC 70771-1082-6
Acyclovir for Injection, USP
Equivalent to
1000 mg/vial
acyclovir
For Intravenous Infusion Only
10 x 1000 mg vials
Rx only
zydus pharmaceuticals
