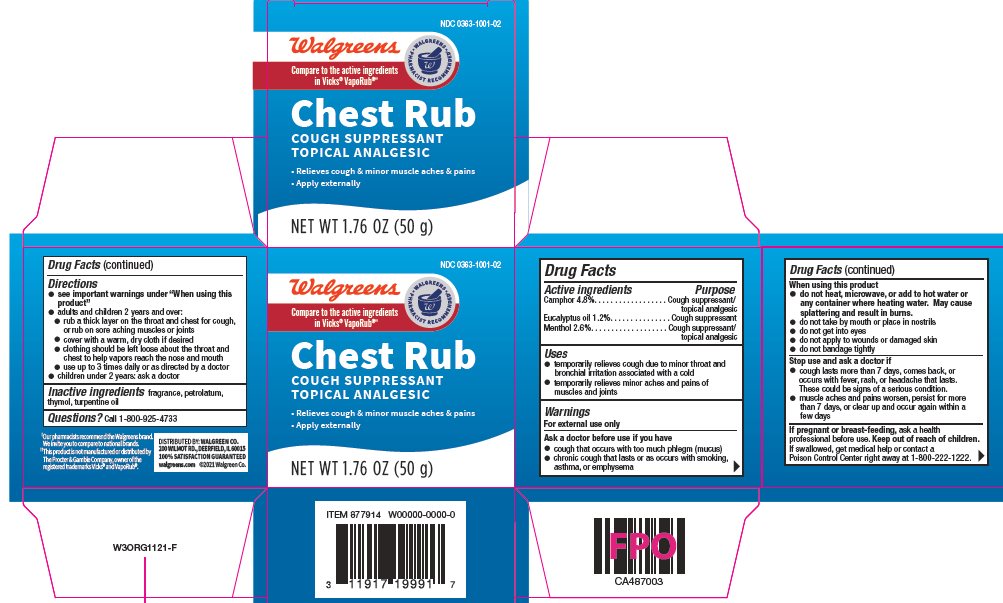
Purpose
Camphor – Cough suppressant/topical analgesic
Eucalyptus oil – Cough suppressant
Menthol – Cough suppressant/topical analgesic
Uses
- temporarily relieves cough due to minor throat and bronchial irritation associated with a cold
- temporarily relieves minor aches and pains of muscles and joints
Warnings
For external use only
Ask a doctor before use if you have
- cough that occurs with too much phlegm (mucus)
- chronic cough that lasts or occurs with smoking, asthma, or emphysema
When using this product
- do not heat, microwave, or add to hot water or any container where heating water. May cause splattering and result in burns.
- do not take by mouth or place in nostrils
- do not get into eyes
- do not apply to wounds or damaged skin
- do not bandage tightly
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away at 1-800-222-1222.
Directions
- see important warning under "When using this product"
- adults and children 2 years and over:
- rub a thick layer on the throat and chest for cough, or rub on sore aching muscles and joints
- cover with a warm, dry cloth if desired
- clothing should be left loose about the throat and chest to help vapors reach the nose and mouth
- use up to 3 times daily, or as directed by a doctor
- children under 2 years: ask a doctor