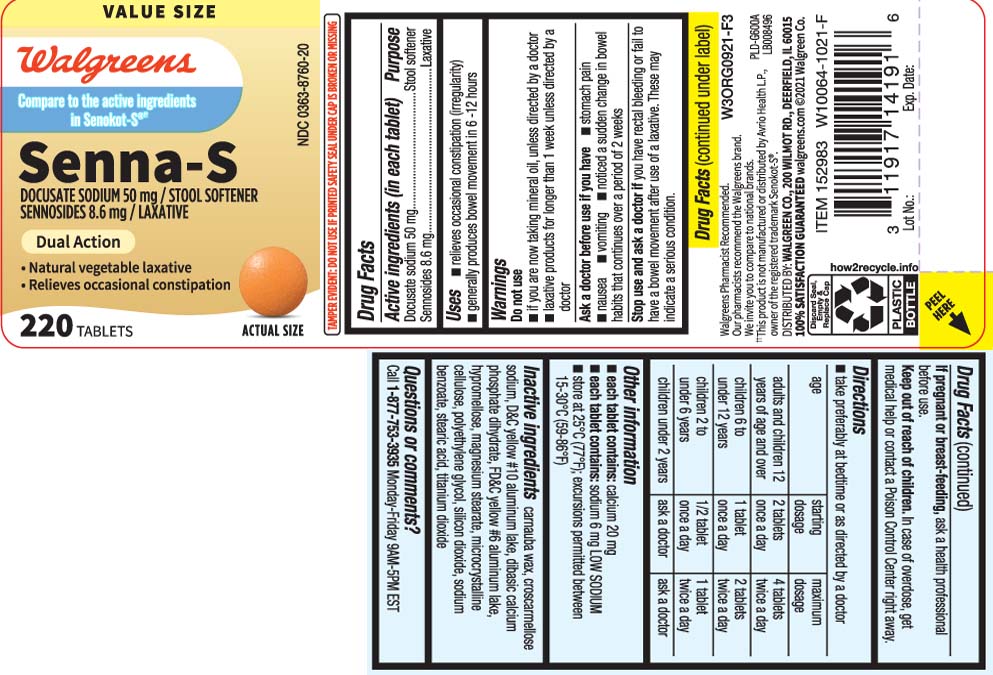
Uses
- relieves occasional constipation (irregularity)
- generally produces bowel movement in 6-12 hours
Warnings
Do not use
- if you are now taking mineral oil, unless directed by a doctor
- laxative products for longer than 1 week unless directed by a doctor
Ask a doctor before use if you have
- stomach pain
- nausea
- vomiting
- noticed a sudden change in bowel habits that continues over a periodof 2 weeks
Directions
- take preferably at bedtime or as directed by a doctor
| age | starting dosage | maximum dosage |
| adults and children 12 years of age or over | 2 tablets once a day | 4 tablets twice a day |
| children 6 to under 12 years | 1 tablet once a day | 2 tablets twice a day |
| children 2 to under 6 years | 1/2 tablet once a day | 1 tablet twice a day |
| children under 2 years | ask a doctor | ask a doctor |
Other information
- each tablet contains: calcium 20 mg
- each tablet contains: sodium 6 mg LOW SODIUM
- store at 25ºC (77ºF); excursions permitted between 15-30ºC (59-86ºF)
Inactive ingredients
carnauba wax, croscarmellose sodium, D&C yellow #10 aluminum lake, dibasic calcium phosphate dihydrate, FD&C yellow #6 aluminum lake, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, silicon dioxide, sodium benzoate, stearic acid, titanium dioxide
Principal Display Panel
Compare to the active ingredients in Senokot-S®††
Senna-S
DOCUSATE SODIUM 50 mg /
STOOL SOFTENER
SENNOSIDES 8.6 mg / LAXATIVE
DUAL ACTION
- Natural Vegetable Laxative
- Gently relieves constipation
TABLETS
††This product is not manufactured or distributed by Avrio Health L.P., owner of the registered trademark Senokot-S®
TAMPER EVIDENT: DO NOT USE IF PRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING.
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION
≠≠This product is not manufactured or distributed by Purdue Products L.P., distributor of Senokot-S®.
DISTRIBUTED BY: WALGREENS CO
200 WILMOT RD., DEERFIELD, IL 60015
walgreens.com