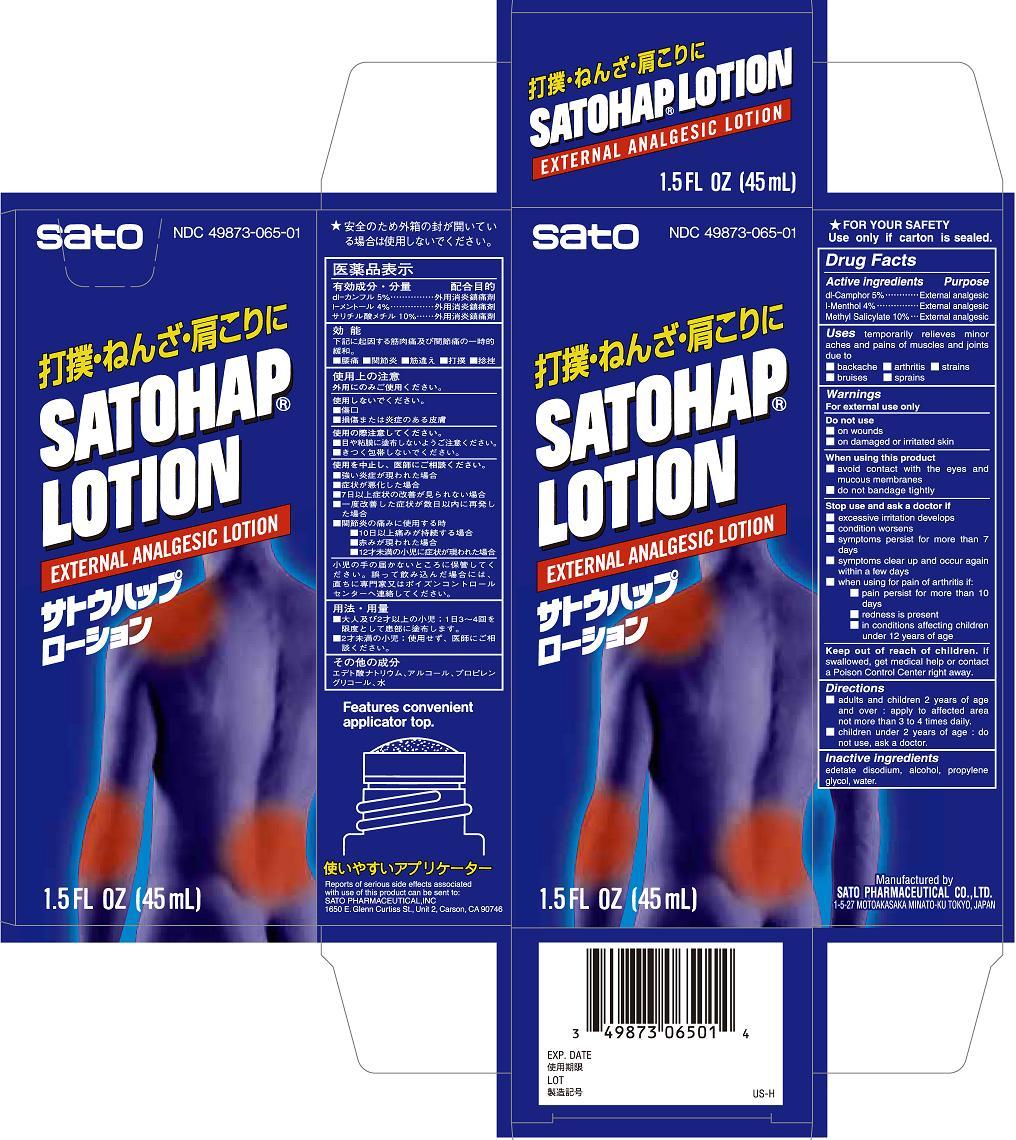
Purpose
dl-Camphor External analgesic
l-Menthol External analgesic
Methyl salicylate External analgesic
Uses
temporarily relieves minor aches and pains of muscles and joints due to
■ backache ■ arthritis ■ strains ■ bruises ■ sprains
Warnings
For external use only
Stop use and ask a doctor if
■ excessive irritation develops
■ condition worsens
■ symptoms persist for more than 7 days
■ symptoms clear up and occur again within a few days
■ when using for pain of arthritis if:
■ pain persist for more than 10 days
■ redness is present
■ in conditions affecting children under 12 years of age