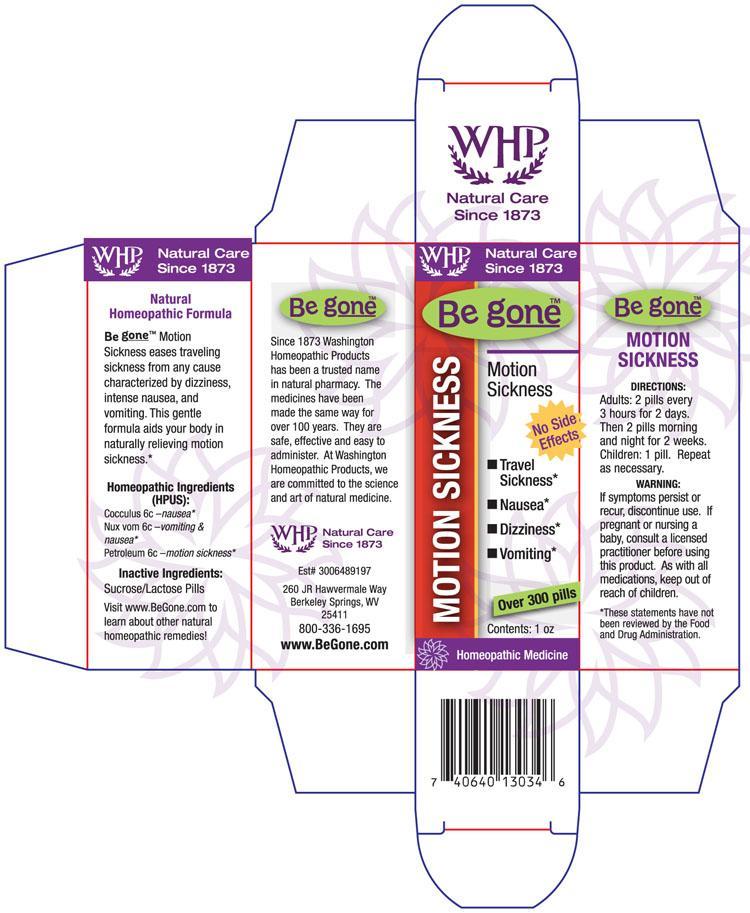
BE GONE MOTION SICKNESS TM- anamirta cocculus seed - strychnos nux-vomica seed - kerosene pellet
Washington Homeopathic Products
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
ACTIVE INGREDIENTS
COCCULUS 6C
NUX VOM 6C
PETROLEUM 6C
USES
To relieve the symptoms of traveling sickness from any cause characterized by dizziness, intense nausea, vomiting.
KEEP OUT OF REACH OF CHILDREN
As with all medications, keep out of reach of children.
INDICATIONS
Indications:
COCCULUS Motion sickness
NUX VOM Vomiting
PETROLEUM Nausea
STOP USE AND ASK DOCTOR
If symptoms persistor recur, discontinue use. If pregnant or nursing a baby, consult a licensed practitioner before using this product.
DIRECTIONS
Adults 2 pills every 3 hours for 2 days. Then 2 pills morning and night for 2 weeks.
Children: 1 pills. Repeat as necessary.
INACTIVE INGREDIENTS
Sucrose/Lactose
PRINCIPAL DISPLAY PANEL
 Be g
oneTM Motion Sickness label
Be g
oneTM Motion Sickness label
 Be g
oneTM Motion Sickness box
Be g
oneTM Motion Sickness box
 Be g
Be g
 Be g
Be g