Immediately nourishes your skin, resulting in a noticeably brighter, softer, and smother complexion.
After cleansing and applying toner, remove sheet mask from packaging and place carefully around eyes and nose. Relax for 2- minutes. Remove mask and discard.
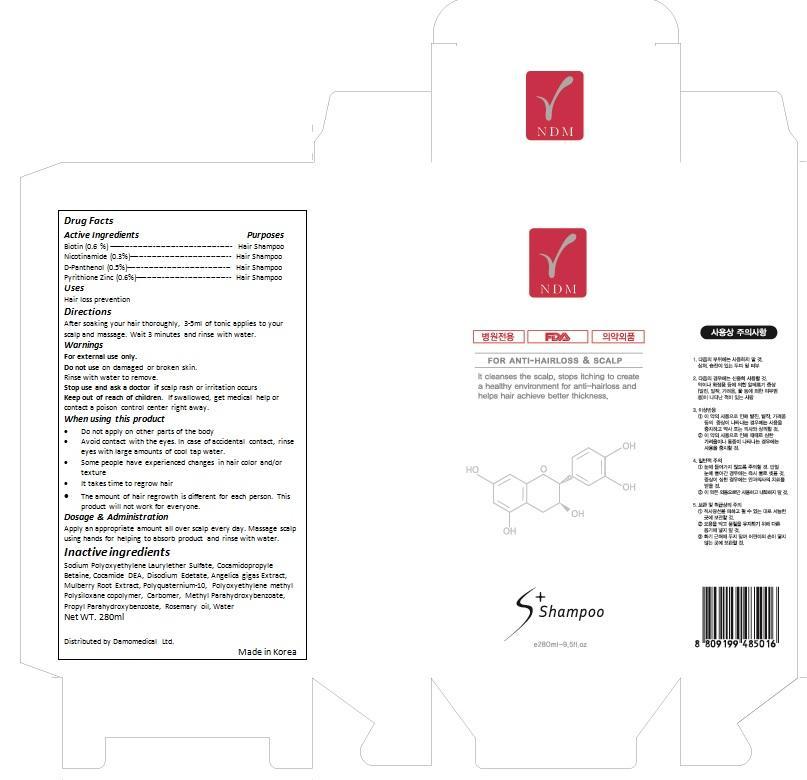
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Water, Butylene Glycol, Dipropylene Glycol, Glycerin, Alcohol, Trehalose, Lavandula Angustifolia (Lavender) Extract(1%), Diethoxyethyl Succinate, Bis-PEG-18 Methyl Ether Dimethyl Silane, Caprylic/Capric Triglyceride, PEG-60 Hydrogenated Castor Oil, Glyceryl Stearate, Sucrose Polystearate, Cetearyl Glucoside, Hydroxyethyl Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Carbomer, Phenoxyethanol, Sophora Angustifolia Root Extract, Lonicera Japonica (Honeysuckle) Flower Extract, Camellia Sinensis Leaf Extract, Behenyl Alcohol, Cetearyl Alcohol, Prunus Persica (Peach) Leaf Extract, Ginkgo Biloba Leaf Extract, Potassium Hydroxide, Fragrance, Ethylhexylglycerin, Scutellaria Baicalensis Root Extract, Hydrogenated Lecithin, Glyceryl Polyacrylate, Disodium EDTA, Hydrolyzed Collagen, Caprylyl Glycol, Glyceryl Caprylate, Sodium Hyaluronate