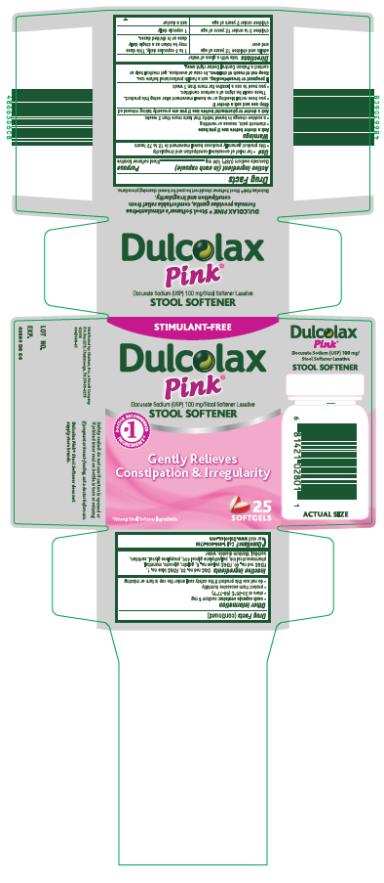
Dulcolax Pink®
Stool Softener
Drug Facts
Active ingredient (in each capsule)
Docusate sodium (USP) 100 mg
Purpose
Stool softener laxative
Use
● for relief of occasional constipation and irregularity
● this product generally produces bowel movement in 12 to 72 hours
Warnings
Do not use
● if you are presently taking mineral oil, unless told to do so by a doctor
Ask a doctor before use if you have
● stomach pain, nausea or vomiting
● a sudden change in bowel habits that lasts more than 2 weeks
Stop use and ask a doctor if
● you have rectal bleeding or no bowel movement after using this product. These could be signs of a serious condition.
● you need to use a laxative for more than 1 week
If pregnant or breast-feeding,
ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
take with a glass of water
adults and children 12 years of age and over
1 to 3 capsules daily. This dose may be taken as a single daily dose or in divided doses.
children 2 to under 12 years of age
1 capsule daily
children under 2 years of age
ask a doctor
Other information
● each capsule contains: sodium 6 mg
● store at 20°-25°C (68°-77°F)
● protect from excessive humidity
● do not use this product if the safety seal under the cap is torn or missing
Inactive ingredients
D&C red no. 33, FD&C blue no. 1, FD&C red no. 40, FD&C yellow no. 6, gelatin, glycerin, mannitol, pharmaceutical ink, polyethylene glycol 400, propylene glycol, sorbitan, sorbitol, titanium dioxide, water
Questions?
Call 1-866-844-2798 or visit www.Dulcolax.com
PRINCIPAL DISPLAY PANEL
Dulcolax
Pink
STOOL SOFTENER
25 SOFTGELS
Chattem, Inc.
