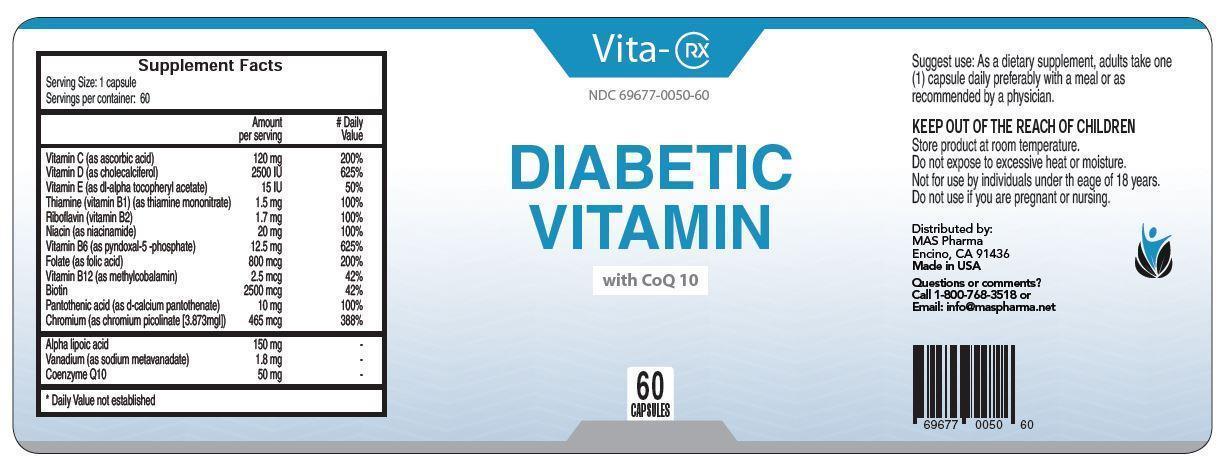
VITA-RX DIABETIC VITAMIN- vitamin c, vitamin d, vitamin e, thiamine, riboflavin, niacin, vitamin b6, folate, vitamin b12, biotin, pantothenic acid, chromium capsule, gelatin coated
Pure Source, LLC
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Vita-Rx
Diabetic Vitamin
with CoQ 10
Suggest use: As a dietary supplement, adults take one (1) capsule daily preferably with a meal or as recommended by a physician.
KEEP OUT OF THE REACH OF CHILDREN
Store product at room temperature.
Do not expose to excessive heat or moisture.
Not for use by individuals under th eage of 18 years.
Do not use if you are pregnant or nursing.
Suggest use: As a dietary supplement, adults take one (1) capsule daily preferably with a meal or as recommended by a physician.