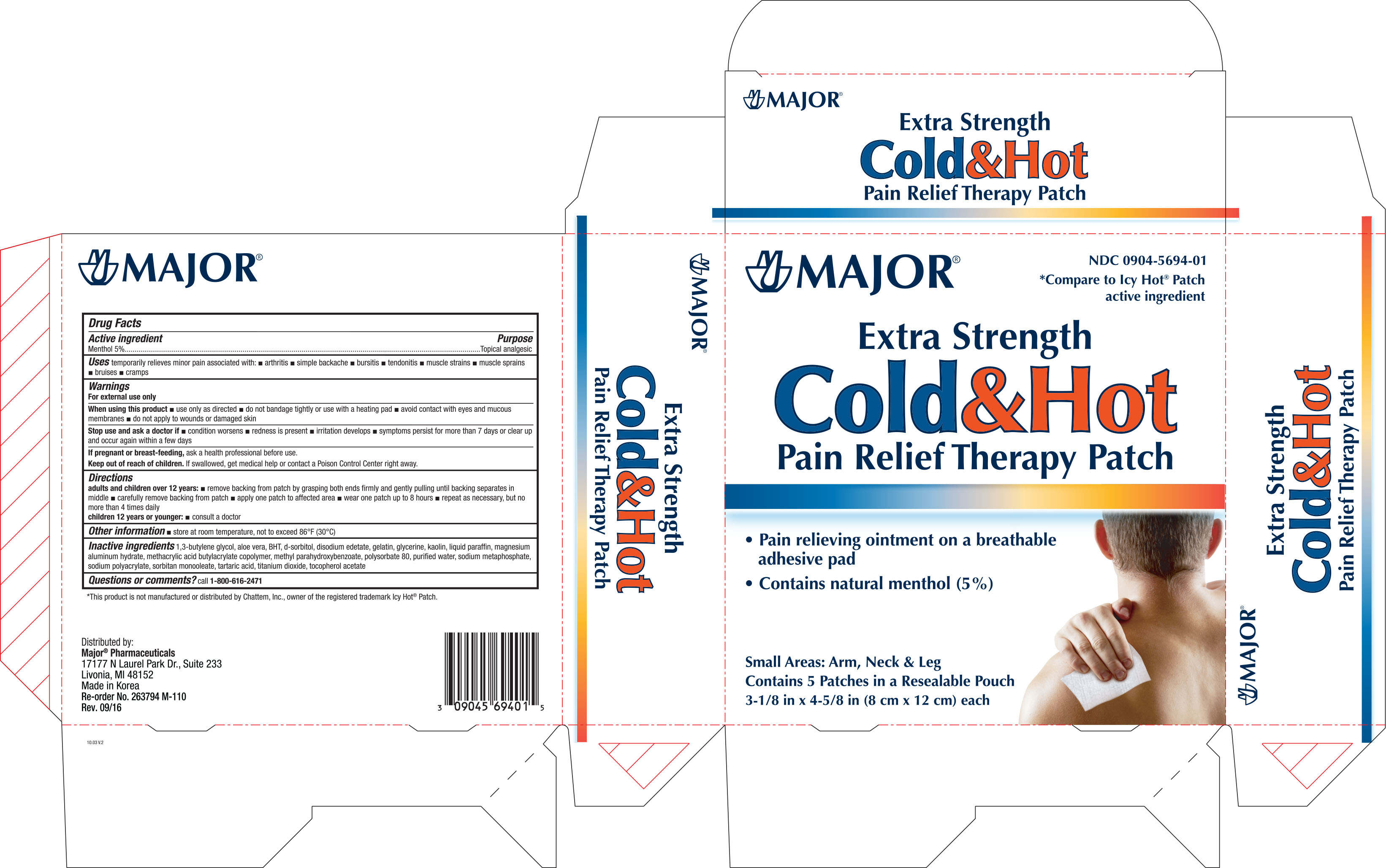
Active ingredient Purpose
Menthol 5%..........................................................................Topical analgesic
Uses
Temporarily relieves minor pain associated with:
- arthritis
- simple backache
- bursitis
- tendonitis
- muscle strains
- muscle sprains
- bruises
- cramps
W hen using this product
- use only as directed
- do not bandage tightly or use with a heating pad
- avoid contact with eyes and mucous membranes
- do not apply to wounds or damaged skins
Stop use and ask a doctor if
- condition worsens
- redness is present
- irritation develops
- symptoms persist for more than 7 days or clear up and occur again within a few days
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- adults and children 12 years:
- remove backing from patch by grasping both ends firmly and gently pulling until backing seperates in middle
- carefully remove backing from patch
- apply one patch to affected area
- wear one patch up to 8 hours
- repeat as necessary, but no more than 4 times daily
children 12 years or younger:
- consult a doctor
Inactive ingredients 1,3-butlene glycol, aloe vera, BHT, d-sorbitol, disodium edetate, gelatin, glycerin, kaolin, liquid paraffin, magnesium aluminum hydrate, methacrylic acid butylacrylate copolymer, methyl parahydroxybenzoate, polysorbate 80, purified water, sodium metaphosphate, sodium polyacrylate, sorbitan monooleate, tartaric acid, titanium dioxide, tocopherol acetate