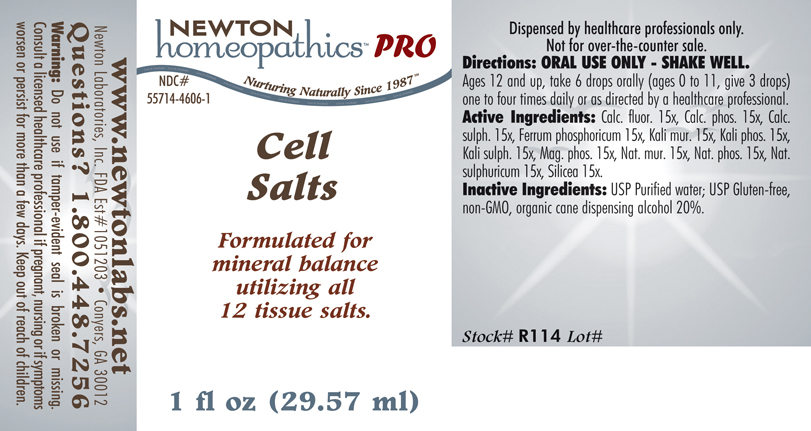
PRODUCT NAME & INDICATIONS SECTION
CELL SALTS Formulated for mineral balance utilizing all 12 tissue salts.
DIRECTION SECTION
Directions: ORAL USE ONLY - SHAKE WELL. Ages 12 and up, take 6 drops orally (ages 0 to 11, give 3 drops) one to four times daily or as directed by a healthcare professional.
ACTIVE INGREDIENT SECTION
Calc. fluor. 15x, Calc. phos. 15x, Calc. sulph. 15x, Ferrum phosphoricum 15x, Kali mur. 15x, Kali phos. 15x, Kali sulph. 15x, Mag. phos. 15x, Nat. mur. 15x, Nat. phos. 15x, Nat. sulphuricum 15x, Silicea 15x.
INACTIVE INGREDIENT SECTION
Inactive Ingredients:USP Purified Water; USP Gluten-free, non-GMO, organic cane dispensing alcohol 20%.
QUESTIONS? SECTION
www.newtonlabs.net Newton Laboratories, Inc. FDA Est # 1051203 -Conyers, GA 30012
Questions? 1.800.448.7256
WARNINGS SECTION
Warning: Do not use if tamper - evident seal is broken or missing. Consult a licensed healthcare professional if pregnant, nursing or if symptoms worsen or persist for more than a few days. Keep out of reach of children.