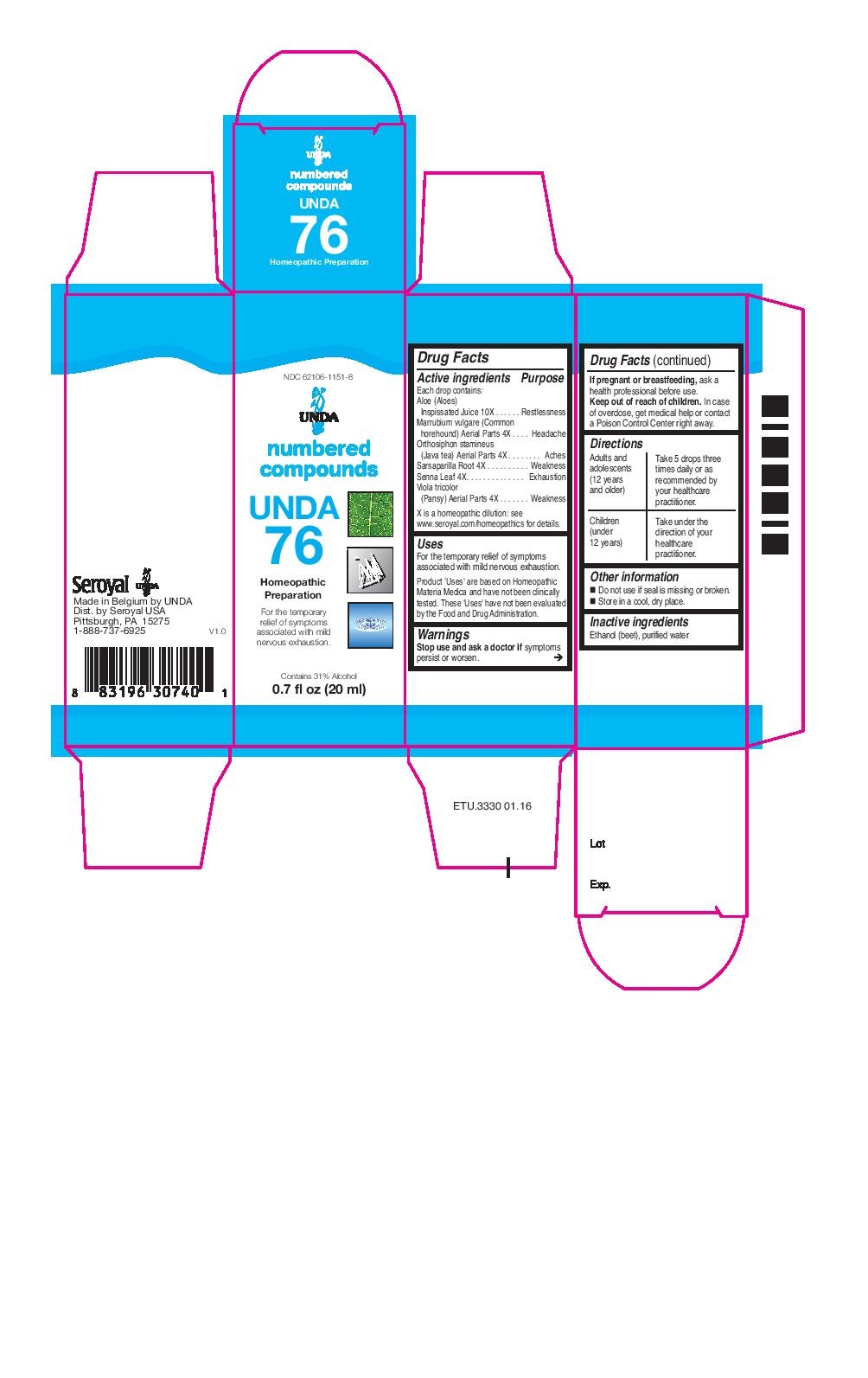
Active ingredients
Each drop contains:
Aloe (Aloes) Inspissated Juice 10X
Marrubium vulgare (Common horehound) Aerial Parts 4X
Orthosiphon stamineus (Java tea) Aerial Parts 4X
Sarsaparilla Root 4X
Senna Leaf 4X
Viola tricolor (Pansy) Aerial Parts 4X
Warnings
Stop use and ask a doctor if symptoms persist or worsen.
If pregnant or breastfeeding, ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
Adults and adolescents (12 years and older)
Take 5 drops three times daily or as recommended by your healthcare practitioner.
Children (under 12 years)
Take under the direction of your healthcare practitioner.
Uses
For the temporary relief of symptoms associated with mild nervous exhaustion.
Directions
Adults and adolescents (12 years and older)
Take 5 drops three times daily or as recommended by your healthcare practitioner.
Children (under 12 years)
Take under the direction of your healthcare practitioner.