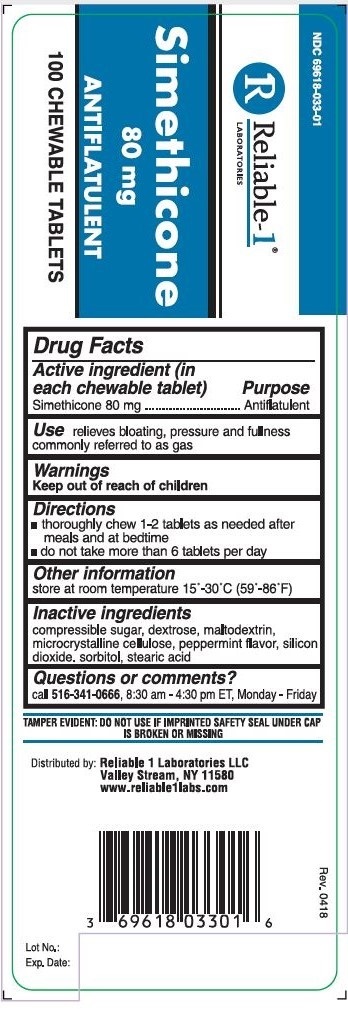
SIMETHICONE 80 MG- simethicone tablet, chewable
Reliable 1 Laboratories LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Use
relieves bloating, pressure and fullness commonly referred to as gas
Warnings
Keep out of reach of children
Keep out of reach of children
Directions
- thoroughly chew 1-2 tablets as needed after meals and at bedtime
- do not take more then 6 tablets per day
Other information
store at room temperature 15°-30°C (59°-86°F)
Inactive ingredients
compressible sugar, dextrose, maltodextrin, micdocrystalline cellulose, peppermint flavor, Silicon dioxide, sorbitol, stearic acid
Questions or comments?
call
516-341-0666, 8:30 am to 4:30 pm ET, Monday - Friday
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
Active ingredient (in each chewable tablet)
Simethicone 80 mg
NDC 69618-033-01
Simethicone 80 mg
ANTIFLATULENT
100 CHEWABLE TABLETS