TULA RADIANT SKIN BRIGHTENING SERUM SKIN TINT SUNSCREEN BROAD SPECTRUM SPF 30 VERY LIGHT NEUTRAL- avobenzone, octisalate serum liquid
TULA RADIANT SKIN BRIGHTENING SERUM SKIN TINT SUNSCREEN BROAD SPECTRUM SPF 30 VERY LIGHT COOL- avobenzone, octisalate serum liquid
TULA RADIANT SKIN BRIGHTENING SERUM SKIN TINT SUNSCREEN BROAD SPECTRUM SPF 30 VERY LIGHT WARM- avobenzone, octisalate serum liquid
TULA RADIANT SKIN BRIGHTENING SERUM SKIN TINT SUNSCREEN BROAD SPECTRUM SPF 30 LIGHT WARM- avobenzone, octisalate serum liquid
TULA RADIANT SKIN BRIGHTENING SERUM SKIN TINT SUNSCREEN BROAD SPECTRUM SPF 30 LIGHT NEUTRAL- avobenzone, octisalate serum liquid
TULA RADIANT SKIN BRIGHTENING SERUM SKIN TINT SUNSCREEN BROAD SPECTRUM SPF 30 LIGHT COOL- avobenzone, octisalate serum liquid
TULA RADIANT SKIN BRIGHTENING SERUM SKIN TINT SUNSCREEN BROAD SPECTRUM SPF 30 LIGHT NEUTRAL-WARM- avobenzone, octisalate serum liquid
TULA RADIANT SKIN BRIGHTENING SERUM SKIN TINT SUNSCREEN BROAD SPECTRUM SPF 30 LIGHT-MEDIUM COOL- avobenzone, octisalate serum liquid
TULA RADIANT SKIN BRIGHTENING SERUM SKIN TINT SUNSCREEN BROAD SPECTRUM SPF 30 LIGHT-MEDIUM WARM- avobenzone, octisalate serum liquid
TULA RADIANT SKIN BRIGHTENING SERUM SKIN TINT SUNSCREEN BROAD SPECTRUM SPF 30 LIGHT-MEDIUM OLIVE- avobenzone, octisalate serum liquid
TULA RADIANT SKIN BRIGHTENING SERUM SKIN TINT SUNSCREEN BROAD SPECTRUM SPF 30 LIGHT-MEDIUM NEUTRAL- avobenzone, octisalate serum liquid
TULA RADIANT SKIN BRIGHTENING SERUM SKIN TINT SUNSCREEN BROAD SPECTRUM SPF 30 MEDIUM WARM- avobenzone, octisalate serum liquid
TULA RADIANT SKIN BRIGHTENING SERUM SKIN TINT SUNSCREEN BROAD SPECTRUM SPF 30 MEDIUM COOL- avobenzone, octisalate serum liquid
TULA RADIANT SKIN BRIGHTENING SERUM SKIN TINT SUNSCREEN BROAD SPECTRUM SPF 30 MEDIUM NEUTRAL- avobenzone, octisalate serum liquid
TULA RADIANT SKIN BRIGHTENING SERUM SKIN TINT SUNSCREEN BROAD SPECTRUM SPF 30 MEDIUM NEUTRAL-COOL- avobenzone, octisalate serum liquid
TULA RADIANT SKIN BRIGHTENING SERUM SKIN TINT SUNSCREEN BROAD SPECTRUM SPF 30 MEDIUM-TAN WARM- avobenzone, octisalate serum liquid
TULA RADIANT SKIN BRIGHTENING SERUM SKIN TINT SUNSCREEN BROAD SPECTRUM SPF 30 MEDIUM-TAN NEUTRAL- avobenzone, octisalate serum liquid
TULA RADIANT SKIN BRIGHTENING SERUM SKIN TINT SUNSCREEN BROAD SPECTRUM SPF 30 MEDIUM-TAN OLIVE- avobenzone, octisalate serum liquid
TULA RADIANT SKIN BRIGHTENING SERUM SKIN TINT SUNSCREEN BROAD SPECTRUM SPF 30 TAN WARM- avobenzone, octisalate serum liquid
TULA RADIANT SKIN BRIGHTENING SERUM SKIN TINT SUNSCREEN BROAD SPECTRUM SPF 30 TAN NEUTRAL-COOL- avobenzone, octisalate serum liquid
TULA RADIANT SKIN BRIGHTENING SERUM SKIN TINT SUNSCREEN BROAD SPECTRUM SPF 30 TAN COOL- avobenzone, octisalate serum liquid
TULA RADIANT SKIN BRIGHTENING SERUM SKIN TINT SUNSCREEN BROAD SPECTRUM SPF 30 TAN-DEEP OLIVE- avobenzone, octisalate serum liquid
TULA RADIANT SKIN BRIGHTENING SERUM SKIN TINT SUNSCREEN BROAD SPECTRUM SPF 30 TAN-DEEP COOL- avobenzone, octisalate serum liquid
TULA RADIANT SKIN BRIGHTENING SERUM SKIN TINT SUNSCREEN BROAD SPECTRUM SPF 30 TAN-DEEP NEUTRAL- avobenzone, octisalate serum liquid
TULA RADIANT SKIN BRIGHTENING SERUM SKIN TINT SUNSCREEN BROAD SPECTRUM SPF 30 DEEP WARM- avobenzone, octisalate serum liquid
TULA RADIANT SKIN BRIGHTENING SERUM SKIN TINT SUNSCREEN BROAD SPECTRUM SPF 30 DEEP NEUTRAL-WARM- avobenzone, octisalate serum liquid
TULA RADIANT SKIN BRIGHTENING SERUM SKIN TINT SUNSCREEN BROAD SPECTRUM SPF 30 DEEP NEUTRAL-COOL- avobenzone, octisalate serum liquid
TULA RADIANT SKIN BRIGHTENING SERUM SKIN TINT SUNSCREEN BROAD SPECTRUM SPF 30 DEEP COOL- avobenzone, octisalate serum liquid
TULA RADIANT SKIN BRIGHTENING SERUM SKIN TINT SUNSCREEN BROAD SPECTRUM SPF 30 DEEP NEUTRAL- avobenzone, octisalate serum liquid
Tula Life LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active Ingredients
Avobenzone 3%
Octisalate 5%
Uses
Helps prevent sunburn • if used as directed with other
sun protection measures (see Directions), decreases the
risk of skin cancer and early skin aging caused by the sun
Warnings
For external use only.
Do not use on damaged or broken skin
When using this product keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if rash occurs
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right
away.
Keep out of reach of children
Keep out of reach of children. If swallowed, get medical help or contact a
Poison Control Center right away.
Directions
Apply liberally 15 minutes before sun exposure.
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF of 15 or higher and other sun protection measures including: limit time in the sun, especially from 10 am – 2 pm, wear long sleeved shirts, pants, hats and sunglasses.
Children under 6 months: Ask a doctor
Inactive Ingredients
Aqua/Water/Eau, C9-12 Alkane , Butyloctyl Salicylate, Caprylic/Capric Triglyceride, Ethylhexyl Palmitate, Triethylhexanoin, Polyglyceryl-6 Polyhydroxystearate, Polyglyceryl-6 Polyricinoleate, Isododecane, Silica, Butylene Glycol, Disteardimonium Hectorite, Trimethylsiloxysilicate, VP/Eicosene Copolymer, Glycerin, Argania Spinosa Kernel Oil, Tocopheryl Acetate, VP/Hexadecene Copolymer, Octyldodecyl Neopentanoate, Sodium Chloride, Magnesium Sulfate, Boron Nitride, Sorbitan Oleate, Polyglycerin-6, Caesalpinia Spinosa Fruit Extract, Octyldodecanol, Safflower oil/ Palm oil aminopropanediol esters , Mica , Lactococcus Ferment Lysate, Alpha-Glucan Oligosaccharide, Hydrogenated Lecithin, Bisabolol, Pentaerythrityl Tetra-di-t-butyl Hydroxyhydrocinnamate, Simethicone, Kappaphycus Alvarezii Extract, Pongamia Glabra Seed Oil , Beta Vulgaris (Beet) Root Extract, Cystoseira Tamariscifolia Extract, Inulin, Niacinamide, Tocopherol, Tetrahexyldecyl Ascorbate, Ascorbyl Palmitate, Lactic Acid, Polymnia Sonchifolia Root Juice, Lactobacillus Ferment, Curcuma Longa (Turmeric) Root Extract, Maltodextrin, Ceramide NP, Dunaliella Salina Extract , Haematococcus Pluvialis Extract , Acacia Seyal Gum Extract, Lactobacillus, Ethylhexylglycerin, Aluminum Hydroxide, Sodium Hyaluronate, Citric Acid, 1,2-Hexanediol, Caprylyl Glycol, Phenoxyethanol, Sodium Benzoate, Potassium Sorbate, Titanium Dioxide (CI 77891), Iron Oxides (CI 77492), Iron Oxides (CI 77491), Iron Oxides (CI 77499).
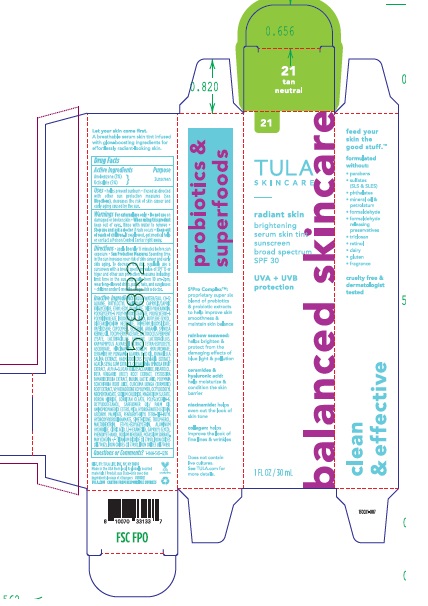
Principal Display Panel
TULA SKINCARE
Radiant Skin Brightening Serum Skin Tint Sunscreen Broad Spectrum SPF 20
UVA + UVB Protection
1FL. OZ/30 mL
Shade 1: Very Light Neutral

Shade 2 Very Light Cool



Shade 3 Very Light Warm


Shade 4 Light Warm


Shade 5 Light Neutral


Shade 6 Light Cool


Shade 7 Light Neutral-Warm


Shade 8 Light-Medium Cool



Shade 9 Light-Medium Warm


Shade 10 Light-Medium Olive



Shade 11 Light-Medium Neutral


Shade 12 Medium Warm


Shade 13 Medium Cool


Shade 14 Medium Neutral


Shade 15 Medium Neutral-Cool


Shade 16 Medium-Tan Warm


Shade 17 Medium-Tan Neutral


Shade 18 Medium-Tan Olive


Shade 19 Tan Warm


Shade 20 Tan Neutral-Cool


Shade 21 Tan Neutral

Shade 22 Tan Cool


Shade 23 Tan-Deep Olive


Shade 24 Tan-Deep Cool


Shade 25 Tan-Deep Neutral


Shade 26 Deep Warm


Shade 27 Deep Neutral-Warm

Shade 28 Deep Neutral-Cool

Shade 29 Deep Cool

Shade 30 Deep Neutral


























































