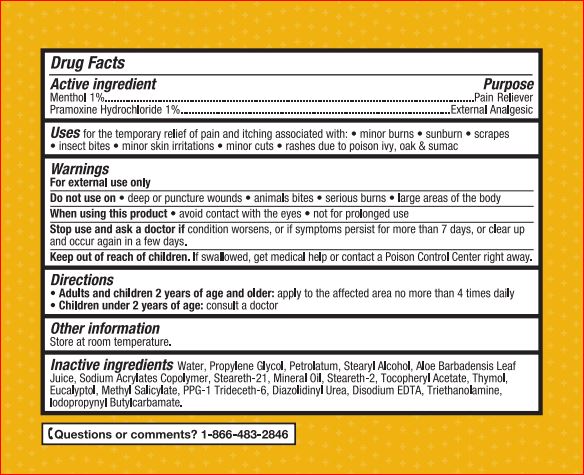
Active ingredients Purpose
Menthol – 1.00% Pain Reliever
Pramoxine Hydrochloride – 1.00% External Analgesic
Uses For temporarily relief of pain and itching associated with:• sunburn • minor burns • scrapes • insect bites • minor skin irritation • minor cuts • rashes due to poison ivy, oak & sumac
Warnings
For external use only
Directions
- Adults and children 2 years of age and older: apply to the affected area no more than 4 times daily
- Children under 2 years of age: consult a doctor
Inactive ingredients
Water, Propylene Glycol, Petrolatum, Stearyl Alcohol, Aloe Barbadensis Leaf Juice, Sodium Acrylates Copolymer, Steareth-21, Mineral Oil,
Steareth-2, Tocopheryl Acetate, Thymol, Eucalyptol, Methyl Salicylate, PPG-1 Trideceth-6, Diazolidinyl Urea, Disodium EDTA, Triethanolamine,
Iodopropynyl Butylcarbamate