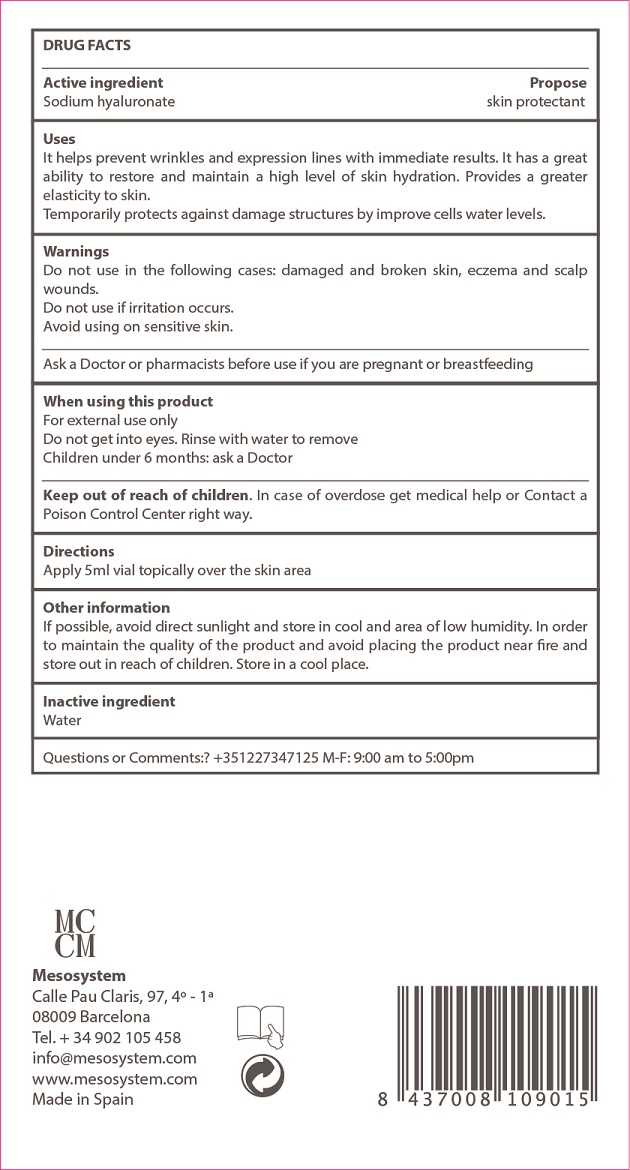
Uses
helps to prevent wrinkles and expression lines with inmediate results. It has a great ability to restore and maintain a high level of skin hydration. Provides a greater elasticity to skin.
. temporarily protects against damage structures by improving cells water levels.
Warnings
For external use only.
Do not use on the following cases: damaged or broken skin, eczema and scalp wounds.
Do not use if irritation occurs
Avoid using on sensitive skins.
When using this product keep our of the eyes. Rinse with water to remove.
Keep out of reach of children.
Children under 6 months: as a doctor.
Other information
. if possible, avoid direct sunlight and stores in a cool area of low humidity. In order to maintain the quality of the product, avoid placing the product near fire and store out of the reach of children.
. store in a cool place
Active Ingredients Purpose
Active Ingredient Purpose
Sodium Hyaluronate.................... Skin Protectant