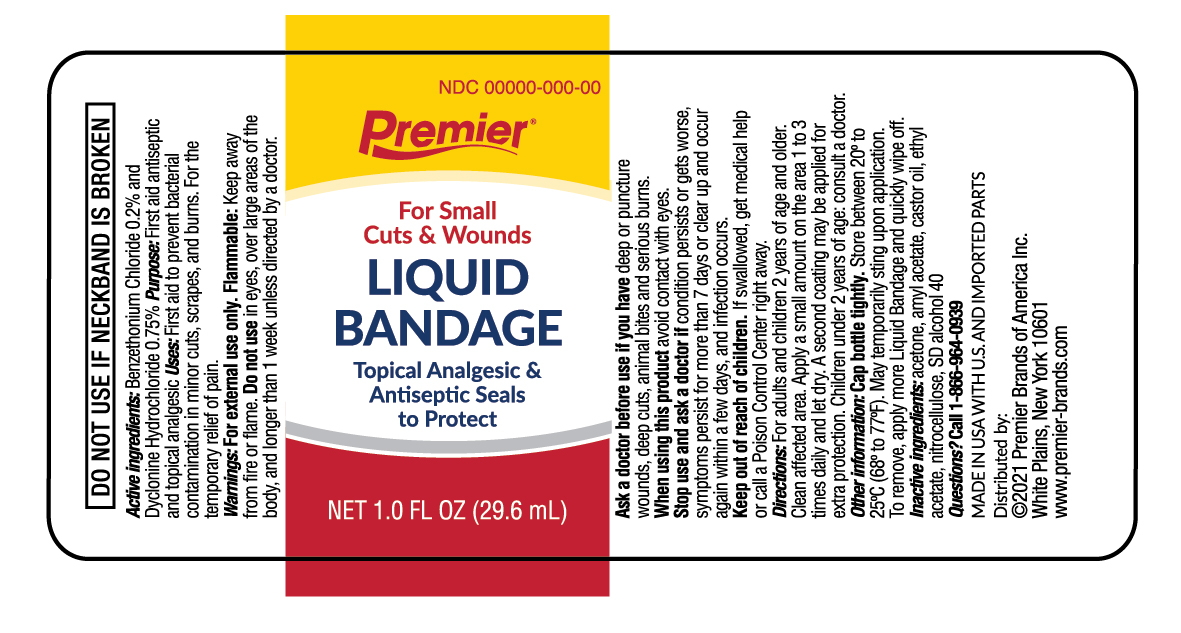
Warnings
For external use only.
Do not use
- in the eyes
- over large areas of the body
- longer than 1 week unless directed by a doctor
- on infected areas or wounds that are draining
- with other first aid products such as lotions and creams
- over sutures
- on mucous membranes
Ask a doctor before use if you have
- deep puncture wounds
- deep cuts
- animal bites
- serious bleeding
- diabetes
- poor circulation
- serious burns
Directions
- Clean affected area
- apply a small amount on the area 1-3 times daily
- let dry
- a second coating may be applied for extra protection
- to remove, apply more Liquid Bandage and quickly wipe off
- finger nail polish remover may dissolve Liquid Bandage
Other information
- cap bottle tightly
- store at room temperature away from heat
- may temporarily sting upon application
- do not allow to come in contact with floors, countertops, or other finished surfaces - will stain
Inactive ingredients
acetone, amyl acetate, castor oil, ethyl acetate, nitrocellulose, SD slcohol 40
Principal Display Panel
Premier
For small Cuts & Wounds
Liquid Bandage
Topical Analgesic & Antiseptic
Prevents infection
Invisible & flexible
Easy to use
NET 0.3 FL OZ (9 mL)
USE LIQUID BANDAGE FOR:
Blisters, chapped & cracked finger tips, hangnails, paper cuts, shaving nicks & help in preventing the formation of calluses.
GREAT FOR:
Athletes, active lifestyles, outdoor sportment & musicians.