SUPPLEMENT FACTS
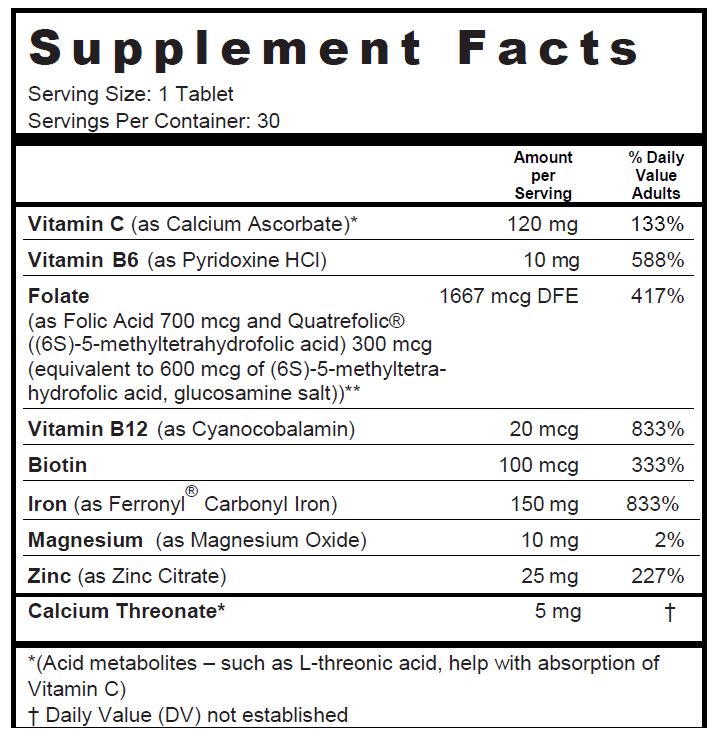
Other Ingredients: Microcrystalline Cellulose, Pregelatinized Starch, di-Calcium Phosphate, Red Color Coating (Hydroxypropylmethyl Cellulose, Polyvinyl Alcohol, FD&C Red #40 Lake, Polyethylene Glycol, Talc, Titanium Dioxide), Croscarmellose Sodium, Stearic Acid, Magnesium Stearate
VEGAN FRIENDLY
GLUTEN-, LACTOSE- AND SUGAR-FREE
CORVITE®150 is a prescription hematinic multivitamin/multimineral dietary supplement used to improve the nutritional status of patients with iron deficiency.
Other Ingredients
Microcrystalline Cellulose, Pregelatinized Starch, di-Calcium Phosphate, Red Color Coating (Hydroxypropylmethyl Cellulose, Polyvinyl Alcohol, FD&C Red #40 Lake, Polyethylene Glycol, Talc, Titanium Dioxide), Croscarmellose Sodium, Stearic Acid, Magnesium Stearate.
Sugar, Lactose and Gluten Free
Vegan Safe
CONTRAINDICATIONS
CORVITE®150 should not be used by patients with a known hypersensitivity to any of the listed ingredients. All iron compounds are contraindicated in patients with hemochromatosis, hemosiderosis, or hemolytica anemias.
WARNINGS
| WARNING: Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. KEEP THIS PRODUCT OUT OF REACH OF CHILDREN. In case of accidental overdose, call a doctor or poison control center immediately. |
PRECAUTIONS
Do not exceed recommended dose. The type of anemia and the underlying cause or causes should be determined before starting therapy with CORVITE®150. Since the anemia may be a result of a systemic disturbance, such as recurrent blood loss, the underlying cause or causes should be corrected, if possible.
Folic Acid: Folic acid alone is an improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where vitamin B12 is deficient. Folic acid in doses above 0.1 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurologic manifestations remain progressive. Allergic sensitization has been reported following both oral and parenteral administration of folic acid.
The patient’s medical conditions and consumption of other drugs, herbs, and/or supplements should be considered.
Pediatric Use: Safety and effectiveness in pediatric populations have not been established.
Geriatric Use: Safety and effectiveness in elderly populations have not been established.
Biotin levels higher than the recommended daily allowance may cause interference with some laboratory tests, including cardiovascular diagnostic tests (e.g. troponin) and hormone tests, and may lead to incorrect test results. Tell your healthcare provider about all prescription and over-the-counter medicines, vitamins, and dietary supplements that you take, including biotin.
DRUG INTERACTIONS
CORVITE® 150 is not recommended for and should not be given to patients receiving levodopa because the action of levodopa is antagonized by pyridoxine. There is a possibility of increased bleeding due to pyridoxine interaction with anticoagulants (e.g., Aspirin, Heparin, Clopidogrel).
ADVERSE REACTIONS
Adverse reactions with iron therapy may include constipation, diarrhea, nausea, vomiting, dark stools and abdominal pain. Adverse reactions with iron therapy are usually transient.
OVERDOSAGE
The clinical course of acute iron overdosage can be variable. Initial symptoms may include abdominal pain, nausea, vomiting, diarrhea, tarry stools, melena, hematemesis, hypotension, tachycardia, metabolic acidosis, hyperglycemia, dehydration, drowsiness, pallor, cyanosis, lassitude, seizures, shock and coma.
DESCRIPTION
CORVITE®150 is a red, oval shaped tablet imprinted with “VP052” on one side and bisected on the other side.
STORAGE
Store at controlled room temperature 15°-30°C (59°-86°F) [See USP]. Protect from light, moisture and excessive heat. Dispense in a tight, light resistant container as defined in the USP using a child-resistant closure.
KEEP THIS PRODUCT OUT OF REACH OF CHILDREN.
**Quatrefolic® is a registered trademark of Gnosis, SpA. U.S. Patent No. 7,947,662.
For use on the order of a healthcare practitioner
Call your doctor about side effects. To report side effects, call Vertical Pharmaceuticals at 1-770-509-4500 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
PLR-CORV150-00001-1 Rev. 12/2021

Distributed by:
Vertical Pharmaceuticals, LLC
Alpharetta, GA 30005
www.verticalpharma.com
