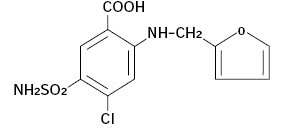
Description:
Furosemide is a potent loop diuretic which is a derivative of anthranilic acid. The structure is:
Chemical Name: 4-Chloro-N-furfuryl-5-sulfamoylanthranilic acid.
Furosemide is pharmacodynamically characterized by the following:
1) It is administered orally. It is easily absorbed from the intestinal tract and begins to act in 30 to 60 minutes after oral administration.1, 2
2) Is a loop diuretic which inhibits reabsorption of sodium and chloride at the ascending loop of Henle in the kidneys, enhancing water excretion.3
3) A dose-response relationship and a ratio of minimum to maximum effective dose range greater than tenfold.1
4) A high degree of efficacy, low inherent toxicity and a high therapeutic index.
Actions:
The therapeutic efficacy of furosemide tablets is from the activity of the intact and unaltered molecule throughout the nephron, inhibiting the reabsorption of sodium not only in the proximal and distal tubule but also in the ascending limb of the loop of Henle. The prompt onset of action is a result of the drug's rapid absorption and a poor lipid solubility. The low lipid solubility and a rapid renal excretion minimize the possibility of its accumulation in tissues and organs or crystalluria. Furosemide tablets have no inhibitory effect on carbonic anhydrase or aldosterone activity in the distal tubule. The drug possesses diuretic activity either in the presence of acidosis or alkalosis.1, 2, 4, 5, 6
Indications:
Dogs - Furosemide tablets are indicated for the treatment of edema (pulmonary congestion, ascites) associated with cardiac insufficiency and acute non-inflammatory tissue edema. In cases of edema involving cardiac insufficiency, the continued use of heart stimulants such as digitalis or its glycosides is indicated. The rationale for efficacious use of diuretic therapy is determined by the clinical pathology producing the edema.
Dosage and administration:
The usual oral dosage of furosemide tablets is 1 to 2 mg/lb body weight (approximately 2.5 to 5 mg/kg). A prompt diuresis usually ensues from the initial treatment.
Administer orally once or twice daily at 6 to 8 hour intervals. The dosage should be adjusted to the individual's response. In severe edematous or refractory cases, the dose may be doubled or increased by increments of 1.0 mg per pound of body weight. The established effective dose should be administered once or twice daily. The daily schedule of administration can be timed to control the period of micturition for the convenience of the client or veterinarian. Mobilization of the edema may be most efficiently and safely accomplished by utilizing an intermittent daily dose schedule, i.e., every other day or 2 to 4 consecutive days weekly.
Diuretic therapy should be discontinued after reduction of the edema, or maintained after determining a carefully programmed dosage schedule to prevent recurrence of edema. For long-term treatment, the dose can generally be lowered after the edema has once been reduced. Re-examination and consultations with the client will enhance the establishment of a satisfactorily programmed dosage schedule. Clinical examination and serum BUN, CO2 and electrolyte determinations should be performed during the early period of therapy and periodically thereafter, especially in refractory cases. Abnormalities should be corrected or the drug temporarily withdrawn.
Dosage: Oral
Dog: One-half to one 50 mg scored tablet per 25 pounds body weight. One 12.5 mg scored tablet per 5 to 10 pounds body weight.
Administer once or twice daily, permitting a 6- to 8-hour interval between treatments. In refractory or severe edematous cases, the dosage may be doubled or increased by increments of 1 mg per pound body weight as recommended in preceding paragraphs, "Dosage and administration".
Contraindications:
Animal reproductive studies have shown that furosemide may cause fetal abnormality and the drug is contraindicated in pregnant animals. Furosemide is contraindicated in anuria, furosemide hypersensitivity, hepatic coma, or during electrolytic imbalances. Monitor serum electrolytes, BUN and CO2 frequently. Monitor serum potassium levels and watch for signs of hypocalcemia.
Corticosteroids cause an additive potassium-depletion effect.
Warnings:
Furosemide tablets are a highly effective diuretic and, if given in excessive amounts, as with any diuretic, may lead to excessive diuresis which could result in electrolyte imbalance, dehydration and reduction of plasma volume, enhancing the risk of circulatory collapse, thrombosis and embolism. Therefore, the animal should be observed for early signs of fluid depletion with electrolyte imbalance, and corrective measures administered. Excessive loss of potassium in patients receiving digitalis or its glycosides may precipitate digitalis toxicity. Caution should be exercised in animals administered potassium-depleting steroids. Correct potassium deficiency with proper dietary supplementation. If animal needs potassium supplements, use oral liquid form, do not use enteric-coated potassium tablets.
The concurrent use of furosemide with some antibiotics may be inadvisable. There is evidence that the drug enhances the nephrotoxic potential of aminoglycosides, cephalosporins and polymyxins and increases the ototoxic effects of all aminoglycosides.
Sulfonamide diuretics have been reported to decrease arterial responsiveness to pressor amines and to enhance the effect of tubocurarine. Caution should be exercised in administering curare or its derivatives to patients undergoing therapy with Furosemide tablets and it is advisable to discontinue Furosemide tablets for one day prior to any elective surgery.
Precautions:
Furosemide tablets are a highly effective diuretic-saluretic which, if given in excessive amounts, may result in dehydration and electrolyte imbalance. Therefore, the dosage and schedule may have to be adjusted to the patient's need. The animal should be observed for early signs of electrolyte imbalance, and corrective measures administered. Early signs of electrolyte imbalance are increased thirst, lethargy, drowsiness or restlessness, fatigue, oliguria, gastrointestinal disturbances and tachycardia. Special attention should be given to potassium levels. Furosemide tablets may lower serum calcium levels and cause tetany in rare cases of animals having an existing hypocalcemic tendency.7, 8, 9, 10, 11
Furosemide tablets are contraindicated in anuria. Therapy should be discontinued in cases of progressive renal disease if increasing azotemia and oliguria occur during the treatment. Sudden alterations of fluid and electrolyte imbalance in an animal with cirrhosis may precipitate hepatic coma, therefore, observation during period of therapy is necessary. In hepatic coma and in states of electrolyte depletion, therapy should not be instituted until the basic condition is improved or corrected. Potassium supplementation may be necessary in cases routinely treated with potassium-depleting steroids.
Active or latent diabetes may on rare occasions be exacerbated by furosemide. Transient loss of auditory capacity has been experimentally produced in cats following intravenous injections of excessive doses of furosemide at a very rapid rate.12, 13, 14
Animal safety:
Furosemide demonstrates a very low order of either acute or chronic toxicity. The drug is rapidly absorbed and excreted by both glomerular filtration and tubular secretion. The rates of excretion are of such magnitude that cumulation of furosemide does not occur despite repeated administrations.15
The main effect observed in clinical toxicity is an abnormality of fluid and electrolyte imbalances. Ototoxicity resulting in transient loss of hearing has been reported with furosemide.15
A safety study was performed in dogs to determine the effects of furosemide tablets at increasing dosages and time elements. The dosage levels were 2 mg/lb body weight (upper recommended dosage), 6 mg/lb body weight (3X upper recommended dosage) and 10 mg/lb body weight (5X upper recommended dosage). The treatment period ranged up to nine days in length. Results demonstrate a mild dehydration at the 5X level with a slight elevation of hemoglobin and hematocrit levels. Serum levels of potassium and chloride were slightly lowered in the higher dosage groups. Cumulative evaluation of the data demonstrates that furosemide tablets are safe when administered at the upper level of the recommended dosage for a duration of nine consecutive days.
To report suspected adverse reactions, to obtain a Safety Data Sheet or for technical assistance, call 1-855-724-3461.
For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS, or www.fda.gov/reportanimalae.
Storage:
Store at 20° to 25°C (68° to 77°F), excursions permitted between 15° and 30°C (between 59° and 86°F).
How supplied:
Furosemide tablets are available in 12.5 mg and 50 mg strength and supplied in bottles containing 500 tablets.
NDC 11695-0090-5 - 12.5 mg – 500 tablets
NDC 11695-0091-5 - 50 mg – 500 tablets
References:
- 1.
- Timmerman, R.J., F.R. Springman and R.K. Thoms, 1964. Evaluation of Furosemide, a New Diuretic Agent. Current Therapeutic Research, 6(2):88-94.
- 2.
- Martindale, The Extra Pharmacopoeia.27th ed. June 1977, The Pharmaceutical Press, London, 556.
- 3.
- Suki, W., F.C. Rector, Jr. and D.W. Seldin, 1965. The Site of Action of Furosemide and Other Sulfonamide Diuretics in the Dog. Journal of Clinical Investigation, 44(9):1458-1469.
- 4.
- Berman, L.B. and A. Ebrahimi. February 1965. Experiences with Furosemide in Renal Disease. Proceedings in the Society for Experimental Biology and Medicine, 118:333-336.
- 5.
- Schmidt, H.A.E. Animal Experiments with S35 Tagged Lasix® in Canine and Ovine. Radio-chemical Pharmacological Laboratory, Farbwerke Hoechst, Frankfurt, West Germany.
- 6.
- Haussler, A. and P. Hajdu. Methods Biological Identification and Results of Studies on Absorption, Elimination and Metabolism. Research Laboratories, Farbwerke Hoechst, Frankfurt, West Germany.
- 7.
- Antoniou, L.D., G.M. Eisner, L.M. Slotkoff and L.S. Lilienfield. December 1967. Sodium and Calcium Transport in the Kidney. Clinical Research, 15(4):476.
- 8.
- Duarte, C.G. April 1967. Effects of Furosemide (F) and Ethacrynic Acid (ETA) on the Renal Clearance of Phosphate (Cp), Ultrafilterable Calcium (CUfCa) and Magnesium (CUfMg). Clinical Research, 15(2):357.
- 9.
- Duarte, C.G. October 1968. Effects of Ethacrynic Acid and Furosemide on Urinary Calcium, Phosphate and Magnesium. Metabolism, 17:867-876.
- 10.
- Nielsen, S.P., O. Andersen and K.E. Steven, 1969. Magnesium and Calcium Metabolism during Prolonged Furosemide (Lasix®) Administration to Normal Rats. Acta Pharmacol, et. Toxicol., 27:469-479.
- 11.
- Reimold, E.W. July 1972. The Effect of Furosemide on Hypercalcemia Due to Dihydrotachysterol. Metabolism, 21(7).
- 12.
- Brown, R.D. and T.W. McElwee, Jr. Effects of Intra-Arterially and Intravenously Administered Ethacrynic Acid and Furosemide on Cochlear N1 in Cats. Toxicology and Applied Pharmacology, 22:589-594, 1972.
- 13.
- Mathog, R.H., W.G. Thomas and W.R. Hudson: Ototoxicity of New and Potent Diuretics. Archives of Otolaryngology, 92(1):7-13, July 1970.
- 14.
- Mathog, R.H. and G.J. Matz: Ototoxic Effects of Ethacrynic Acid. Annals of Otolaryngology, Vol. 81, 1972.
- 15.
- Gilman, A.G., L.S. Goodman and A. Gilman, 1980. The Pharmacological Basis of Therapeutics,Sixth ed., MacMillan Publishing Co., Inc., New York, NY, 903-907.
Distributed by:
Covetrus North America
400 Metro Place North
Dublin, OH 43017
covetrus.com
AH-Furosemide tablets-02
REV: 0819
673816-06/673918-06
87018261/87018237
Principal Display Panel - Container Label, 12.5 mg, 500 tablets
covetrus
NDC 11695-0090-5
Furosemide Tablets 12.5 mg
A diuretic-saluretic for prompt relief of edema.
Caution:Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Approved by FDA under NADA # 129-034
500 Tablets
Principal Display Panel - Container Label, 50 mg, 500 tablets
covetrus
NDC 11695-0091-5
Furosemide Tablets50 mg
A diuretic-saluretic for prompt relief of edema.
Furosemide is not USP for dissolution.
Caution:Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Approved by FDA under NADA # 129-034
500 Tablets