| Supplement Facts | ||
|---|---|---|
| Serving Size: 1 Tablet | ||
| Amount Per Serving | % DV* | |
|
||
| Vitamin A (Palmitate) | 4000 IU | 50% |
| Vitamin C (Ascorbic Acid) | 120 mg | 200% |
| Vitamin D (Cholecalciferol) | 400 IU | 100% |
| Vitamin E (dl - Alpha Tocopheryl Acetate) | 30 IU | 100% |
| Vitamin B-1 (Thiamine Mononitrate) | 1.8 mg | 106% |
| Vitamin B-2 (Riboflavin) | 1.7 mg | 85% |
| Niacin (Niacinamide) | 20 mg | 100% |
| Vitamin B-6 (Pyridoxine HCl) | 2.6 mg | 104% |
| Folic Acid | 0.8 mg | 100% |
| Vitamin B-12 (Cyanocobalamin) | 8 mcg | 100% |
| Calcium (Calcium Carbonate) | 200 mg | 15% |
| Iron (Ferrous Fumarate) | 28 mg | 156% |
| Zinc (Oxide) | 25 mg | 167% |
Other ingredients: cellulose, croscarmellose sodium, magnesium stearate, hypromellose, PEG, mineral oil, titanium dioxide, talc, FD&C Red #40, FD&C Yellow #6, FD&C Blue #2
INDICATIONS
For use as a dietary supplement prior to conception, during pregnancy and during the postnatal period, for both the nursing and non-nursing mother.
DIRECTIONS
For adults, one tablet daily, preferably with food. Ask your health care provider before taking any supplements or medications.
| WARNING: Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under six. KEEP THIS PRODUCT OUT OF REACH OF CHILDREN In case of accidental overdose, call a doctor or Poison Control Center immediately. |
Store at room temperature (59° - 86°F).
REV 0615
DIST. BY: GERI-CARE
PHARMACEUTICALS CORP.
1650 63rd Street
Brooklyn, NY 11204
PRINCIPAL DISPLAY PANEL - 100 Tablet Bottle Label
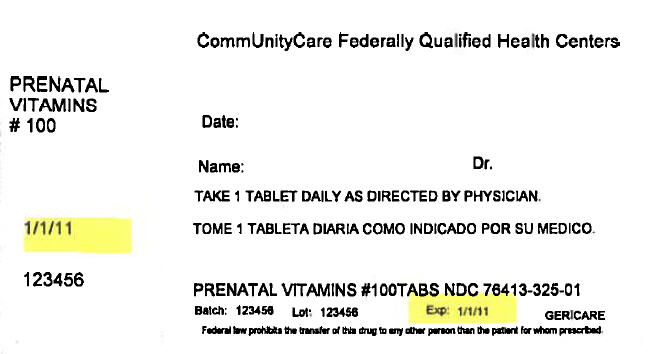
CommUnityCare Federally Qualified Health Centers
PRENATAL
VITAMINS
# 100
Date:
Name:
Dr.
TAKE 1 TABLET DAILY AS DIRECTED BY PHYSICIAN.
1/1/11
123456
PRENATAL VITAMINS #100TABS NDC 76413-325-01
Batch:
123456
Lot:
123456
Exp:
1/1/11
GERICARE
Federal law prohibits the transfer of this drug to any other person than the patient for whom prescribed.