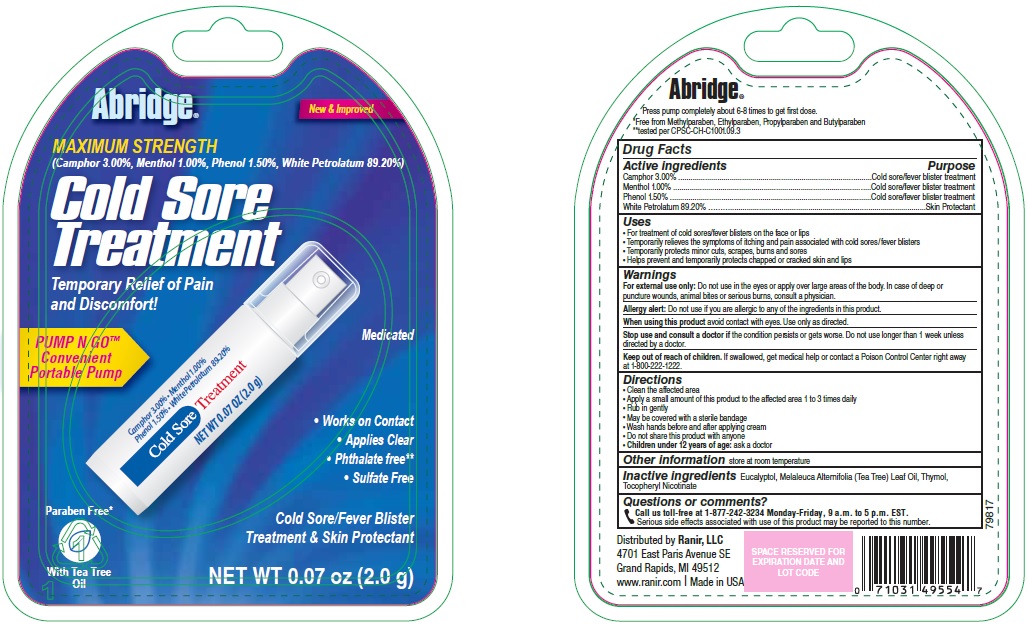
Uses
- •
- For treatment of cold sores/fever blisters on the face or lips
- •
- Temporarily relieves the symptoms of itching and pain associated with cold sores/fever blisters
- •
- Temporarily protects minor cuts, scrapes, burns and sores
- •
- Helps prevent and temporarily protects chapped or cracked skin and lips
Warnings
For external use only: Do not use in the eyes or apply over large areas of the body. In case of deep or puncture wounds, animal bites or serious burns, consult a physician.
Allergy alert: Do not use if you are allergic to any of the ingredients in this product.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away at 1-800-222-1222.
Directions
- •
- Clean the affected area
- •
- Apply a small amount of this product to the affected area 1 to 3 times daily
- •
- Rub in gently
- •
- May be covered with a sterile bandage
- •
- Wash hands before and after applying cream
- •
- Do not share this product with anyone
- •
- Children under 12 years of age: ask a doctor
Inactive ingredients
Eucalyptol, Melaleuca Alternifolia (Tea Tree) Leaf Oil, Thymol, Tocopheryl Nicotinate
Questions or comments?
Call us toll-free at 1-877-242-3234 Monday-Friday, 9 a.m. to 5 p.m. EST.
Serious side effects associated with use of this product may be reported to this number.
Package/Label Principal Display Panel
New & Improved
MAXIMUM STRENGTH
(Camphor 3.00%, Menthol 1.00%, Phenol 1.50%, White Petrolatum 89.20%
Cold Sore Treatment
Temporary Relief of Pain and Discomfort!
Medicated
PUMP N GO™
Convenient Portable Pump
Works on Contact
Applies Clear
Phthalate free
Sulfate Free
Paraben Free
Cold Sore/Fever Blister Treatment & Skin Protectant
With Tea Tree Oil
NET WT 0.07 oz (2.0 g)