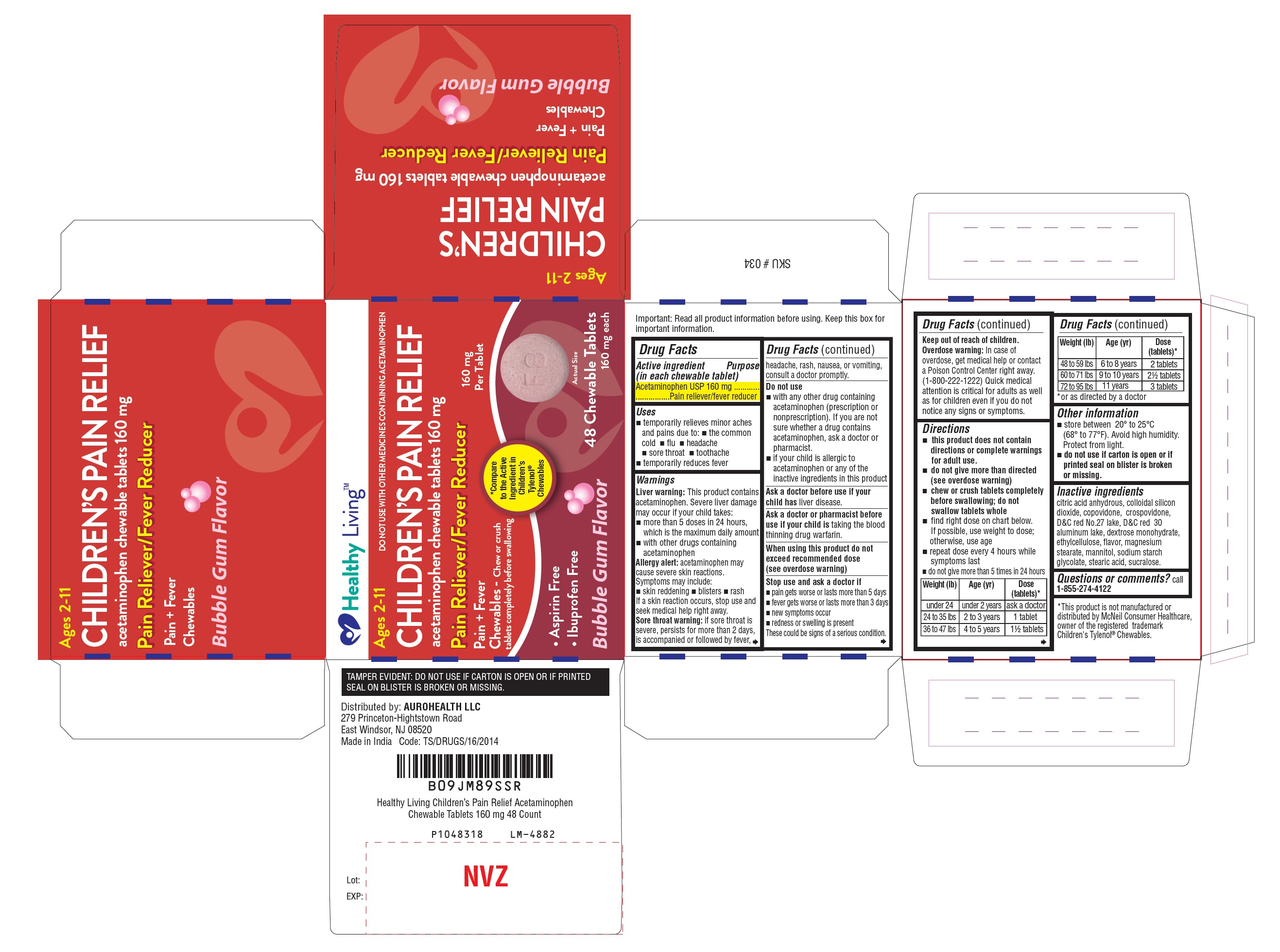
Uses
- temporarily relieves minor aches and pains due to:
- the common cold
- flu
- headache
- soar throat
- toothache
- temporarily reduces fever
Warnings
Liver warning:This product contains acetaminophen. Severe liver damage may occur if your child takes
- more than 5 doses in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
Allergy alert:acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Sore throat warning:
if sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if your child is allergic to acetaminophen or any of the inactive ingredients in this product
Stop use and ask a doctor if
- pain gets worse or lasts more than 5 days
- fever gets worse or lasts more than 3 days
- new symptoms occur
- redness or swelling is present
These could be signs of a serious condition.
Keep out of reach of children.
Overdose warning:In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
Quick medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
Directions
- this product does not contain directions or complete warnings for adult use.
- do not give more than directed (see overdose warning)
- chew or crush tablets completely before swallowing; do not swallow tablets whole
- find right dose on chart below. If possible, use weight to dose; otherwise, use age.
- repeat dose every 4 hours while symptoms last
- do not give more than 5 times in 24 hours
| Weight (lb)
| Age (yr)
| Dose (tablets)*
|
| under 24
| under 2 years
| ask a doctor
|
| 24 to 35 lbs
| 2 to 3 years
| 1 tablet
|
| 36 to 47 lbs
| 4 to 5 years
| 1½ tablets
|
| 48 to 59 lbs
| 6 to 8 years
| 2 tablets
|
| 60 to 71 lbs
| 9 to 10 years
| 2½ tablets
|
| 72 to 95 lbs
| 11 years
| 3 tablets
|
* or as directed by a doctor
Other information
- store between 20° to 25°C (68° to 77°F). Avoid high humidity. Protect from light.
- do not use if carton is open or if printed seal on blister is broken or missing.
Inactive ingredients
citric acid anhydrous, colloidal silicon dioxide, copovidone, crospovidone, D&C red No.27 lake, D&C red 30 aluminum lake, dextrose monohydrate, ethylcellulose, flavor, magnesium stearate, mannitol, sodium starch glycolate, stearic acid, sucralose.
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL 160 mg (48 Chewable Tablets Blister Carton Label)
Healthy Living
TM
Ages 2-11 DO NOT USE WITH OTHER MEDICINES CONTAINING ACETAMINOPHEN
CHILDREN’S PAIN RELIEF
acetaminophen chewable tablets 160 mg
Pain Reliever / Fever Reducer
160 mg
per Tablet
Pain + Fever
Chewables - Chew or crush
tablets completely before swallowing
Compare
to the active
ingredient in
Children's
Tylenol
®
Chewables
• Aspirin Free
• Ibuprofen Free
Bubble Gum Flavor Actual Size
48 Chewable Tablets
160 mg each