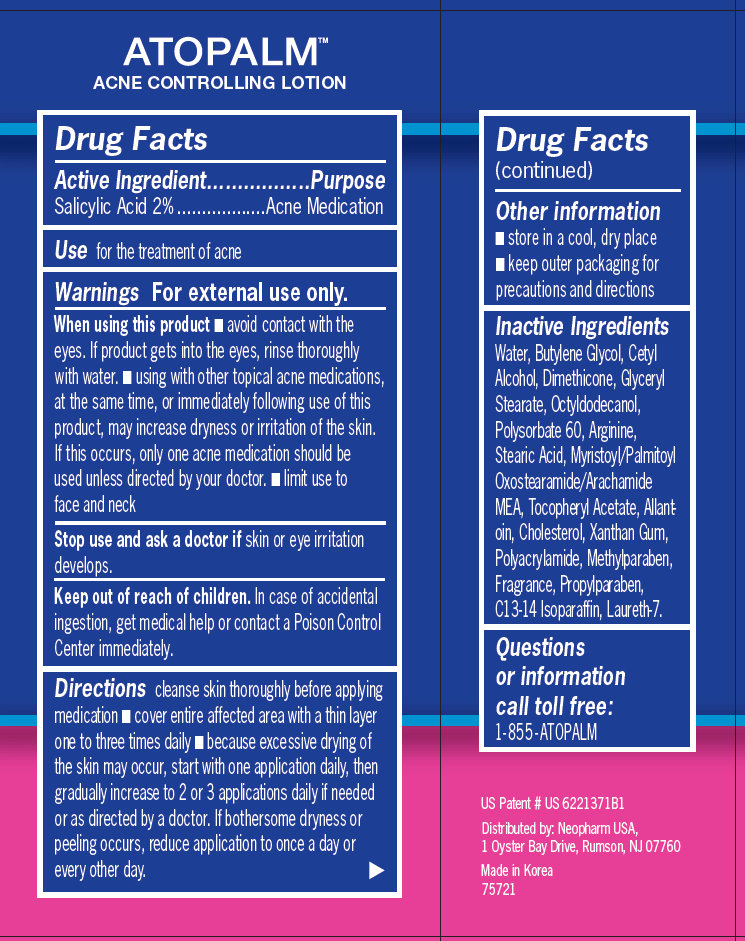
Active Ingredient
Active Ingredient..................................Purpose
Salicylic Acid 2% ..................Acne Medication
When using this product
- avoid contact with the eyes. If product gets into the eyes, rinse thoroughly with water. - using with other topical acne medications, at the same time, or immediately following use of this product, may increase dryness or irritation of the skin. If this occurs, only one acne medication should be used unless directed by your doctor. - limit use to face and neck
Ask Doctor
In case of accidental ingestion, get medical help or contact a Poison Control Center immediately.
Directions
cleanse skin thoroughly before applying medication - cover entire affected area with a thin layer one to three times daily - because excessive drying of the skin may occur, start with one application daily, then gradually increase to 2 or 3 applications daily if needed or as directed by a doctor. If bothersome dryness or peeling occurs, reduce application to once a day or every other day.
Other information
- store in a cool, dry place - keep outer packaging for precautions and directions
Inactive Ingredients
Water, Butylene Glycol, Cetyl Alcohol, Dimethicone, Glyceryl Stearate, Octyldodecanol, Polysorbate 60, Arginine, Stearic Acid, Myristoyl/Palmitoyl Oxostearamide/Arachamide MEA, Tocopheryl Acetate, Allantoin, Cholesterol, Xanthan Gum, Polyacrylamide, Methylparaben, Fragrance, Propylparaben, C13-14 Isoparaffin, Laureth-7.
Description
US Patent # US 6221371B1 Distributed by: Neopharm USA, 1 Oyster Bay Drive, Rumson, NJ 07760 Made in Korea 75721 Quick absorbing all over face lotion has a salicylic based formula that works through the night to help visibly reduce redness and reduce the size of existing pimples.
ATOPALM ACNE CONTROLLING LOTION With US Patented MLE Technology With 2% Salicylic Acid-Acne Medication to help reduce the appearance of redness and pimple size overnight 1.3 fl oz /38.4 ml Drug Facts
Active Ingredient............................. ....................Purpose
Salicylic Acid 2% ................................. Acne Medication
Use for the treatment of acne Warnings For external use only. When using this product - avoid contact with the eyes. If product gets into the eyes, rinse thoroughly with water. - using with other topical acne medications, at the same time, or immediately following use of this product, may increase dryness or irritation of the skin. If this occurs, only one acne medication should be used unless directed by your doctor. - limit use to face and neck Stop use and ask a doctor if skin or eye irritation develops. Keep out of reach of children. In case of accidental ingestion, get medical help or contact a Poison Control Center immediately. Directions cleanse skin thoroughly before applying medication - cover entire affected area with a thin layer one to three times daily - because excessive drying of the skin may occur, start with one application daily, then gradually increase to 2 or 3 applications daily if needed or as directed by a doctor. If bothersome dryness or peeling occurs, reduce application to once a day or every other day. Other information - store in a cool, dry place - keep outer packaging for precautions and directions Questions or information call toll free: 1-855-ATOPALM Distributed by: Neopharm USA, 1 Oyster Bay Drive, Rumson, NJ 07760 Made in Korea 75721
ATOPALM ACNE CONTROLLING LOTION US Patent # US 6221371B1 Distributed by: Neopharm USA, 1 Oyster Bay Drive, Rumson, NJ 07760 Made in Korea 75721
ATOPALM ACNE CONTROLLING LOTION Dermatologist Tested ATOPALM ACNE CONTROLLING LOTION ATOPALM ACNE CONTROLLING LOTION 2% Salicylic Acid Acne Medication Quick absorbing all over face lotion has a salicylic based formula that works through the night to help visibly reduce redness and reduce the size of existing pimples. With US Patented M L E Technology Helps Visibly Reduce Redness and Reduce Pimple Size Overnight 1.3 fl oz /38.4 ml 8 57811 00221 7