DESCRIPTION
Acyclovir is a synthetic nucleoside analogue active against herpes viruses. Acyclovir capsules and tablets are formulations for oral administration.
Each 200 mg capsule contains 200 mg of acyclovir, USP and the inactive ingredients colloidal silicon dioxide, magnesium stearate, pregelatinized starch and sodium lauryl sulfate. In addition, each of the empty gelatin capsules contains gelatin and titanium dioxide and the following colorant agents: FD&C Blue No. 1 and FD&C Red No. 3. The imprinting ink contains the following: black iron oxide, D&C Yellow No. 10 Aluminum Lake, FD&C Blue No. 1 Aluminum Lake, FD&C Blue No. 2 Aluminum Lake, FD&C Red No. 40 Aluminum Lake, pharmaceutical glaze and propylene glycol.
Each 800 mg tablet contains 800 mg of acyclovir, USP and the inactive ingredients croscarmellose sodium, magnesium stearate, microcrystalline cellulose, povidone, and sodium lauryl sulfate.
Each 400 mg tablet contains 400 mg of acyclovir, USP and the inactive ingredients croscarmellose sodium, magnesium stearate, microcrystalline cellulose, povidone, and sodium lauryl sulfate.
Acyclovir, USP is a white to off-white, crystalline powder with a molecular formula C8H11N5O3 and a molecular weight of 225. The maximum solubility in water at 37°C is 2.5 mg/mL. The pka’s of acyclovir are 2.27 and 9.25.
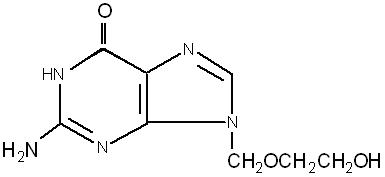
The chemical name of acyclovir is 2-amino-1,9-dihydro-9-[(2-hydroxyethoxy)methyl]-6H-purin-6-one; it has the following structural formula:
VIROLOGY
Mechanism of Antiviral Action
Acyclovir is a synthetic purine nucleoside analogue with in vitro and in vivo inhibitory activity against herpes simplex virus types 1 (HSV-1), 2 (HSV-2), and varicella-zoster virus (VZV).
The inhibitory activity of acyclovir is highly selective due to its affinity for the enzyme thymidine kinase (TK) encoded by HSV and VZV. This viral enzyme converts acyclovir into acyclovir monophosphate, a nucleotide analogue. The monophosphate is further converted into diphosphate by cellular guanylate kinase and into triphosphate by a number of cellular enzymes. In vitro, acyclovir triphosphate stops replication of herpes viral DNA. This is accomplished in three ways: 1) competitive inhibition of viral DNA polymerase, 2) incorporation into and termination of the growing viral DNA chain, and 3) inactivation of the viral DNA polymerase. The greater antiviral activity of acyclovir against HSV compared to VZV is due to its more efficient phosphorylation by the viral TK.
Antiviral Activities
The quantitative relationship between the in vitro susceptibility of herpes viruses to antivirals and the clinical response to therapy has not been established in humans, and virus sensitivity testing has not been standardized. Sensitivity testing results, expressed as the concentration of drug required to inhibit by 50% the growth of virus in cell culture (IC50), vary greatly depending upon a number of factors. Using plaque-reduction assays, the IC50 against herpes simplex virus isolates ranges from 0.02 to 13.5 mcg/mL for HSV-1 and from 0.01 to 9.9 mcg/mL for HSV-2. The IC50 for acyclovir against most laboratory strains and clinical isolates of VZV ranges from 0.12 to 10.8 mcg/mL. Acyclovir also demonstrates activity against the Oka vaccine strain of VZV with a mean IC50 of 1.35 mcg/mL.
Drug Resistance
Resistance of HSV and VZV to acyclovir can result from qualitative and quantitative changes in the viral TK and/or DNA polymerase. Clinical isolates of HSV and VZV with reduced susceptibility to acyclovir have been recovered from immunocompromised patients, especially with advanced HIV infection. While most of the acyclovir-resistant mutants isolated thus far from immunocompromised patients have been found to be TK-deficient mutants, other mutants involving the viral TK gene (TK partial and TK altered) and DNA polymerase have been isolated. TK-negative mutants may cause severe disease in infants and immunocompromised adults. The possibility of viral resistance to acyclovir should be considered in patients who show poor clinical response during therapy.
CLINICAL PHARMACOLOGY
Pharmacokinetics
The pharmacokinetics of acyclovir after oral administration have been evaluated in healthy volunteers and in immunocompromised patients with herpes simplex or varicella-zoster virus infection. Acyclovir pharmacokinetic parameters are summarized in Table 1.
| Parameter | Range |
| Plasma protein binding | 9% to 33% |
| Plasma elimination half-life | 2.5 to 3.3 hr |
| Average oral bioavailability | 10% to 20% |
In one multiple-dose, crossover study in healthy subjects (n = 23), it was shown that increases in plasma acyclovir concentrations were less than dose proportional with increasing dose, as shown in Table 2. The decrease in bioavailability is a function of the dose and not the dosage form.
| Parameter | 200 mg | 400 mg | 800 mg |
| Cssmax | 0.83 mcg/mL | 1.21 mcg/mL | 1.61 mcg/mL |
| Csstrough | 0.46 mcg/mL | 0.63 mcg/mL | 0.83 mcg/mL |
There was no effect of food on the absorption of acyclovir (n = 6); therefore, acyclovir capsules and tablets may be administered with or without food.
The only known urinary metabolite is 9-[(carboxymethoxy)methyl] guanine.
Special Populations
Adults with Impaired Renal Function
The half-life and total body clearance of acyclovir are dependent on renal function. A dosage adjustment is recommended for patients with reduced renal function (see DOSAGE AND ADMINISTRATION).
Geriatrics
Acyclovir plasma concentrations are higher in geriatric patients compared to younger adults, in part due to age related changes in renal function. Dosage reduction may be required in geriatric patients with underlying renal impairment (see PRECAUTIONS: Geriatric Use).
Drug Interactions
Coadministration of probenecid with intravenous acyclovir has been shown to increase the mean acyclovir half-life and the area under the concentration-time curve. Urinary excretion and renal clearance were correspondingly reduced.
Clinical Trials
Initial Genital Herpes
Double-blind, placebo-controlled studies have demonstrated that orally administered acyclovir significantly reduced the duration of acute infection and duration of lesion healing. The duration of pain and new lesion formation was decreased in some patient groups.
Recurrent Genital Herpes
Double-blind, placebo-controlled studies in patients with frequent recurrences (six or more episodes per year) have shown that orally administered acyclovir given daily for 4 months to 10 years prevented or reduced the frequency and/or severity of recurrences in greater than 95% of patients.
In a study of patients who received acyclovir 400 mg twice daily for 3 years, 45%, 52%, and 63% of patients remained free of recurrences in the first, second, and third years, respectively. Serial analyses of the 3-month recurrence rates for the patients showed that 71% to 87% were recurrence free in each quarter.
Herpes Zoster Infections
In a double-blind, placebo-controlled study of immunocompetent patients with localized cutaneous zoster infection, acyclovir (800 mg 5 times daily for 10 days) shortened the times to lesion scabbing, healing, and complete cessation of pain, and reduced the duration of viral shedding and the duration of new lesion formation.
In a similar double-blind, placebo-controlled study, acyclovir (800 mg 5 times daily for 7 days) shortened the times to complete lesion scabbing, healing, and cessation of pain, reduced the duration of new lesion formation, and reduced the prevalence of localized zoster-associated neurologic symptoms (paresthesia, dysesthesia, or hyperesthesia).
Treatment was begun within 72 hours of rash onset and was most effective if started within the first 48 hours.
Adults greater than 50 years of age showed greater benefit.
Chickenpox
Three randomized, double-blind, placebo-controlled trials were conducted in 993 pediatric patients aged 2 to 18 years with chickenpox. All patients were treated within 24 hours after the onset of rash. In two trials, acyclovir was administered at 20 mg/kg 4 times daily (up to 3200 mg per day) for 5 days. In the third trial, doses of 10, 15, or 20 mg/kg were administered 4 times daily for 5 to 7 days. Treatment with acyclovir shortened the time to 50% healing, reduced the maximum number of lesions, reduced the median number of vesicles, decreased the median number of residual lesions on day 28, and decreased the proportion of patients with fever, anorexia, and lethargy by day 2. Treatment with acyclovir did not affect varicella-zoster virus-specific humoral or cellular immune responses at one month or one year following treatment.
INDICATIONS AND USAGE
Herpes Zoster Infections
Acyclovir is indicated for the acute treatment of herpes zoster (shingles).
CONTRAINDICATIONS
Acyclovir is contraindicated for patients who develop hypersensitivity to acyclovir or valacyclovir.
WARNINGS
Acyclovir capsules and tablets are intended for oral ingestion only. Renal failure, in some cases resulting in death, has been observed with acyclovir therapy (see ADVERSE REACTIONS: Observed During Clinical Practice and OVERDOSAGE). Thrombotic thrombocytopenic purpura/hemolytic uremic syndrome (TTP/HUS), which has resulted in death, has occurred in immunocompromised patients receiving acyclovir therapy.
PRECAUTIONS
Dosage adjustment is recommended when administering acyclovir to patients with renal impairment (see DOSAGE AND ADMINISTRATION). Caution should also be exercised when administering acyclovir to patients receiving potentially nephrotoxic agents since this may increase the risk of renal dysfunction and/or the risk of reversible central nervous system symptoms such as those that have been reported in patients treated with intravenous acyclovir. Adequate hydration should be maintained.
Information for Patients
Patients are instructed to consult with their physician if they experience severe or troublesome adverse reactions, they become pregnant or intend to become pregnant, they intend to breast-feed while taking orally administered acyclovir, or they have any other questions. Patients should be advised to maintain adequate hydration.
Herpes Zoster
There are no data on treatment initiated more than 72 hours after onset of the zoster rash. Patients should be advised to initiate treatment as soon as possible after a diagnosis of herpes zoster.
Genital Herpes Infections
Patients should be informed that acyclovir is not a cure for genital herpes. There are no data evaluating whether acyclovir will prevent transmission of infection to others. Because genital herpes is a sexually transmitted disease, patients should avoid contact with lesions or intercourse when lesions and/or symptoms are present to avoid infecting partners. Genital herpes can also be transmitted in the absence of symptoms through asymptomatic viral shedding. If medical management of a genital herpes recurrence is indicated, patients should be advised to initiate therapy at the first sign or symptom of an episode.
Chickenpox
Chickenpox in otherwise healthy children is usually a self limited disease of mild to moderate severity. Adolescents and adults tend to have more severe disease. Treatment was initiated within 24 hours of the typical chickenpox rash in the controlled studies, and there is no information regarding the effects of treatment begun later in the disease course.
Carcinogenesis, Mutagenesis, Impairment of Fertility
The data presented below include references to peak steady-state plasma acyclovir concentrations observed in humans treated with 800 mg given orally 5 times a day (dosing appropriate for treatment of herpes zoster) or 200 mg given orally 5 times a day (dosing appropriate for treatment of genital herpes). Plasma drug concentrations in animal studies are expressed as multiples of human exposure to acyclovir at the higher and lower dosing schedules (see CLINICAL PHARMACOLOGY: Pharmacokinetics).
Acyclovir was tested in lifetime bioassays in rats and mice at single daily doses of up to 450 mg/kg administered by gavage. There was no statistically significant difference in the incidence of tumors between treated and control animals, nor did acyclovir shorten the latency of tumors. Maximum plasma concentrations were 3 to 6 times human levels in the mouse bioassay and 1 to 2 times human levels in the rat bioassay.
Acyclovir was tested in 16 in vitro and in vivo genetic toxicity assays. Acyclovir was positive in five of the assays.
Acyclovir did not impair fertility or reproduction in mice (450 mg/kg/day, p.o.) or in rats (25 mg/kg/day, s.c.). In the mouse study, plasma levels were 9 to 18 times human levels, while in the rat study, they were 8 to 15 times human levels. At higher doses (50 mg/kg/day, s.c.) in rats and rabbits (11 to 22 and 16 to 31 times human levels, respectively) implantation efficacy, but not litter size, was decreased. In a rat perinatal and postnatal study at 50 mg/kg/day, s.c., there was a statistically significant decrease in group mean numbers of corpora lutea, total implantation sites, and live fetuses.
No testicular abnormalities were seen in dogs given 50 mg/kg/day, IV for one month (21 to 41 times human levels) or in dogs given 60 mg/kg/day orally for one year (6 to 12 times human levels). Testicular atrophy and aspermatogenesis were observed in rats and dogs at higher dose levels.
Pregnancy
Teratogenic Effects
Pregnancy Category B
Acyclovir administered during organogenesis was not teratogenic in the mouse (450 mg/kg/day, p.o.), rabbit (50 mg/kg/day, s.c. and IV), or rat (50 mg/kg/day, s.c.). These exposures resulted in plasma levels 9 and 18, 16 and 106, and 11 and 22 times, respectively, human levels.
There are no adequate and well controlled studies in pregnant women. A prospective epidemiologic registry of acyclovir use during pregnancy was established in 1984 and completed in April 1999. There were 749 pregnancies followed in women exposed to systemic acyclovir during the first trimester of pregnancy resulting in 756 outcomes. The occurrence rate of birth defects approximates that found in the general population. However, the small size of the registry is insufficient to evaluate the risk for less common defects or to permit reliable or definitive conclusions regarding the safety of acyclovir in pregnant women and their developing fetuses. Acyclovir should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
Acyclovir concentrations have been documented in breast milk in two women following oral administration of acyclovir and ranged from 0.6 to 4.1 times corresponding plasma levels. These concentrations would potentially expose the nursing infant to a dose of acyclovir up to 0.3 mg/kg/day. Acyclovir should be administered to a nursing mother with caution and only when indicated.
Pediatric Use
Safety and effectiveness of oral formulations of acyclovir in pediatric patients younger than 2 years of age have not been established.
Geriatric Use
Of 376 subjects who received acyclovir in a clinical study of herpes zoster treatment in immunocompetent subjects ≥ 50 years of age, 244 were 65 and over while 111 were 75 and over. No overall differences in effectiveness for time to cessation of new lesion formation or time to healing were reported between geriatric subjects and younger adult subjects. The duration of pain after healing was longer in patients 65 and over. Nausea, vomiting, and dizziness were reported more frequently in elderly subjects. Elderly patients are more likely to have reduced renal function and require dose reduction. Elderly patients are also more likely to have renal or CNS adverse events. With respect to CNS adverse events observed during clinical practice, somnolence, hallucinations, confusion, and coma were reported more frequently in elderly patients (see CLINICAL PHARMACOLOGY, ADVERSE REACTIONS: Observed During Clinical Practice, and DOSAGE AND ADMINISTRATION).
ADVERSE REACTIONS
Herpes Simplex
Short-term Administration
The most frequent adverse events reported during clinical trials of treatment of genital herpes with acyclovir 200 mg administered orally 5 times daily every 4 hours for 10 days were nausea and/or vomiting in 8 of 298 patient treatments (2.7%). Nausea and/or vomiting occurred in 2 of 287 (0.7%) patients who received placebo.
Long-term Administration
The most frequent adverse events reported in a clinical trial for the prevention of recurrences with continuous administration of 400 mg (two 200 mg capsules) 2 times daily for one year in 586 patients treated with acyclovir were nausea (4.8%) and diarrhea (2.4%). The 589 control patients receiving intermittent treatment of recurrences with acyclovir for one year reported diarrhea (2.7%), nausea (2.4%), and headache (2.2%).
Herpes Zoster
The most frequent adverse event reported during three clinical trials of treatment of herpes zoster (shingles) with 800 mg of oral acyclovir 5 times daily for 7 to 10 days in 323 patients was malaise (11.5%). The 323 placebo recipients reported malaise (11.1%).
Chickenpox
The most frequent adverse event reported during three clinical trials of treatment of chickenpox with oral acyclovir at doses of 10 to 20 mg/kg 4 times daily for 5 to 7 days or 800 mg 4 times daily for 5 days in 495 patients was diarrhea (3.2%). The 498 patients receiving placebo reported diarrhea (2.2%).
Observed During Clinical Practice
In addition to adverse events reported from clinical trials, the following events have been identified during post-approval use of acyclovir. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These events have been chosen for inclusion due to either their seriousness, frequency of reporting, potential causal connection to acyclovir, or a combination of these factors.
Nervous
Aggressive behavior, agitation, ataxia, coma, confusion, decreased consciousness, delirium, dizziness, dysarthria, encephalopathy, hallucinations, paresthesia, psychosis, seizure, somnolence, tremors. These symptoms may be marked, particularly in older adults or in patients with renal impairment (see PRECAUTIONS).
Hematologic and Lymphatic
Anemia, leukocytoclastic vasculitis, leukopenia, lymphadenopathy, thrombocytopenia.
Hepatobiliary Tract and Pancreas
Elevated liver function tests, hepatitis, hyperbilirubinemia, jaundice.
Skin
Alopecia, erythema multiforme, photosensitive rash, pruritus, rash, Stevens-Johnson Syndrome, toxic epidermal necrolysis, urticaria.
Urogenital
Renal failure, renal pain (may be associated with renal failure), elevated blood urea nitrogen, elevated creatinine, hematuria (see WARNINGS).
OVERDOSAGE
Overdoses involving ingestion of up to 100 capsules (20 g) have been reported. Adverse events that have been reported in association with overdosage include agitation, coma, seizures, and lethargy. Precipitation of acyclovir in renal tubules may occur when the solubility (2.5 mg/mL) is exceeded in the intratubular fluid. Overdosage has been reported following bolus injections or inappropriately high doses and in patients whose fluid and electrolyte balance were not properly monitored. This has resulted in elevated BUN and serum creatinine and subsequent renal failure. In the event of acute renal failure and anuria, the patient may benefit from hemodialysis until renal function is restored (see DOSAGE AND ADMINISTRATION).
DOSAGE AND ADMINISTRATION
Genital Herpes
Chronic Suppressive Therapy for Recurrent Disease
400 mg 2 times daily for up to 12 months, followed by reevaluation. Alternative regimens have included doses ranging from 200 mg 3 times daily to 200 mg 5 times daily.
The frequency and severity of episodes of untreated genital herpes may change over time. After one year of therapy, the frequency and severity of the patient’s genital herpes infection should be reevaluated to assess the need for continuation of therapy with acyclovir.
Treatment of Chickenpox
Children (2 Years of Age and Older)
20 mg/kg per dose orally 4 times daily (80 mg/kg/day) for 5 days. Children over 40 kg should receive the adult dose for chickenpox.
Adults and Children Over 40 kg
800 mg 4 times daily for 5 days.
Intravenous acyclovir is indicated for the treatment of varicella-zoster infections in immunocompromised patients.
When therapy is indicated, it should be initiated at the earliest sign or symptom of chickenpox. There is no information about the efficacy of therapy initiated more than 24 hours after onset of signs and symptoms.
Patients with Acute or Chronic Renal Impairment
In patients with renal impairment, the dose of acyclovir capsules and tablets should be modified as shown in Table 3:
| Normal Dosage Regimen | Creatinine Clearance (mL/min/1.73m2 ) | Adjusted Dosage Regimen | |
| Dose (mg) | Dosing Interval | ||
| 200 mg every 4 hours | > 10 0 to 10 | 200 200 | every 4 hours, 5x daily every 12 hours |
| 400 mg every 12 hours | > 10 0 to 10 | 400 200 | every 12 hours every 12 hours |
| 800 mg every 4 hours | > 25 10 to 25 0 to 10 | 800 800 800 | every 4 hours, 5x daily every 8 hours every 12 hours |
Hemodialysis
For patients who require hemodialysis, the mean plasma half-life of acyclovir during hemodialysis is approximately 5 hours. This results in a 60% decrease in plasma concentrations following a 6 hour dialysis period. Therefore, the patient’s dosing schedule should be adjusted so that an additional dose is administered after each dialysis.
HOW SUPPLIED
Acyclovir Capsules, USP are available containing 200 mg of acyclovir, USP.
The 200 mg capsule is a hard-shell gelatin capsule with a lavender opaque cap and a lavender opaque body axially printed with MYLAN over 2200 in black ink on both the cap and body. The capsule is filled with white to off-white powder. They are available as follows:
| Bottles of 25 | NDC 54868-3996-0 |
| Bottles of 30 | NDC 54868-3996-2 |
| Bottles of 50 | NDC 54868-3996-3 |
| Bottles of 60 | NDC 54868-3996-5 |
Acyclovir Tablets, USP are available containing 400 mg or 800 mg of acyclovir, USP.
The 400 mg tablets are white round, unscored tablets debossed with M over 253 on one side of the tablet and blank on the other side. They are available as follows:
| Bottles of 10 | NDC 54868-3997-3 |
| Bottles of 20 | NDC 54868-3997-2 |
| Bottles of 30 | NDC 54868-3997-0 |
| Bottles of 40 | NDC 54868-3997-4 |
| Bottles of 60 | NDC 54868-3997-5 |
| Bottles of 100 | NDC 54868-3997-1 |
The 800 mg tablets are white oval, unscored tablets debossed with MYLAN on one side of the tablet and 302 on the other side. They are available as follows:
| Bottles of 20 | NDC 54868-3998-3 |
| Bottles of 30 | NDC 54868-3998-0 |
| Bottles of 35 | NDC 54868-3998-6 |
| Bottles of 40 | NDC 54868-3998-4 |
| Bottles of 50 | NDC 54868-3998-1 |
| Bottles of 60 | NDC 54868-3998-8 |
| Bottles of 100 | NDC 54868-3998-5 |
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.]
Protect from moisture.
Dispense in a tight, light-resistant container as defined in the USP using a child-resistant closure.
Manufactured in India by:
Mylan Laboratories Limited
Hyderabad—500 034, India
Code No. MH/DRUGS/25/NKD/89
Manufactured for:
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505 U.S.A.
REVISED FEBRUARY 2012
MX:ACYCT:R4
75004819
Distributed and Repackaged by:
Physicians Total Care, Inc.
Tulsa, Oklahoma 74146
PRINCIPAL DISPLAY PANEL - 200 mg capsules
Acyclovir
Capsules, USP
200 mg
Rx only
Each capsule contains:
Acyclovir, USP ................ 200 mg
Usual Dosage: See accompanying
prescribing information.
Keep this and all medication out of
the reach of children.
Store at 20° to 25°C (68° to 77°F).
[See USP Controlled Room Temperature.]
Protect from moisture.
Dispense in a tight, light-resistant container
as defined in the USP using a child-resistant
closure.
Keep container tightly closed.

PRINCIPAL DISPLAY PANEL - 400 mg tablets
Acyclovir
Tablets, USP
400 mg
Rx only
Each capsule contains:
Acyclovir, USP ................ 400 mg
Usual Dosage: See accompanying
prescribing information.
Keep this and all medication out of
the reach of children.
Store at 20° to 25°C (68° to 77°F).
[See USP Controlled Room Temperature.]
Protect from moisture.
Dispense in a tight, light-resistant container
as defined in the USP using a child-resistant
closure.
Keep container tightly closed.

PRINCIPAL DISPLAY PANEL - 800 mg tablets
Acyclovir
Tablets, USP
800 mg
Rx only
Each capsule contains:
Acyclovir, USP ................ 800 mg
Usual Dosage: See accompanying
prescribing information.
Keep this and all medication out of
the reach of children.
Store at 20° to 25°C (68° to 77°F).
[See USP Controlled Room Temperature.]
Protect from moisture.
This container is not intended for dispensing
for household use.
Dispense in a tight, light-resistant container
as defined in the USP using a child-resistant
closure.
Keep container tightly closed.
