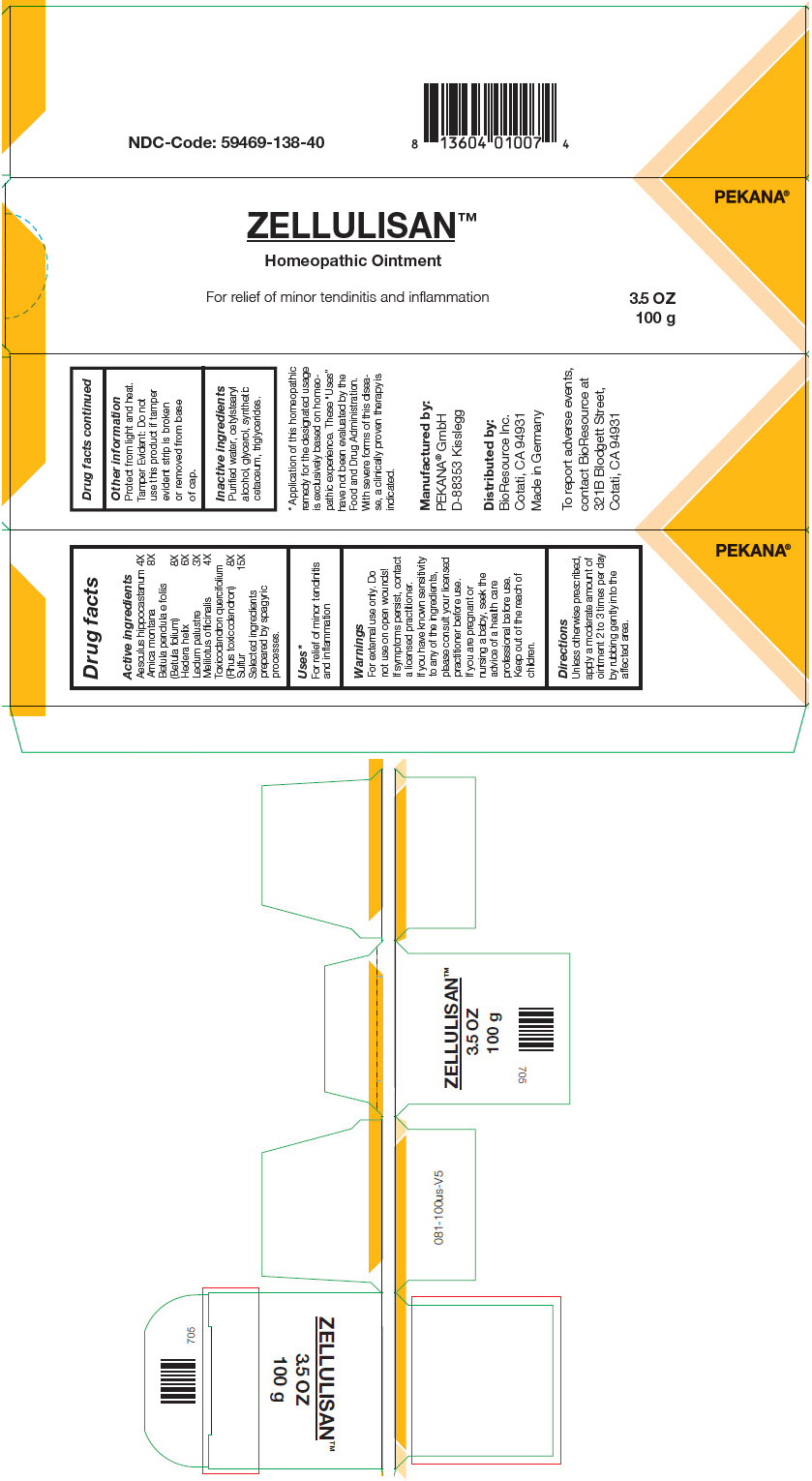
| Active ingredients | |
|---|---|
| Aesculus hippocastanum | 4X |
| Arnica montana | 8X |
| Betula pendula e foliis (Betula folium) | 8X |
| Hedera helix | 6X |
| Ledum palustre | 3X |
| Melilotus officinalis | 4X |
| Toxicodendron quercifolium (Rhus toxicodendron) | 8X |
| Sulfur | 15X |
Selected ingredients prepared by spagyric processes.
Uses1
- 1
- Application of this homeopathic remedy for the designated usage is exclusively based on homeopathic experience. These "Uses" have not been evaluated by the Food and Drug Administration. With severe forms of this disease, a clinically proven therapy is indicated.
Warnings
For external use only. Do not use on open wounds! If symptoms persist, contact a licensed practitioner. If you have known sensitivity to any of the ingredients, please consult your licensed practitioner before use. If you are pregnant or nursing a baby, seek the advice of a health care professional before use.
Directions
Unless otherwise prescribed, apply a moderate amount of ointment 2 to 3 times per day by rubbing gently into the affected area.