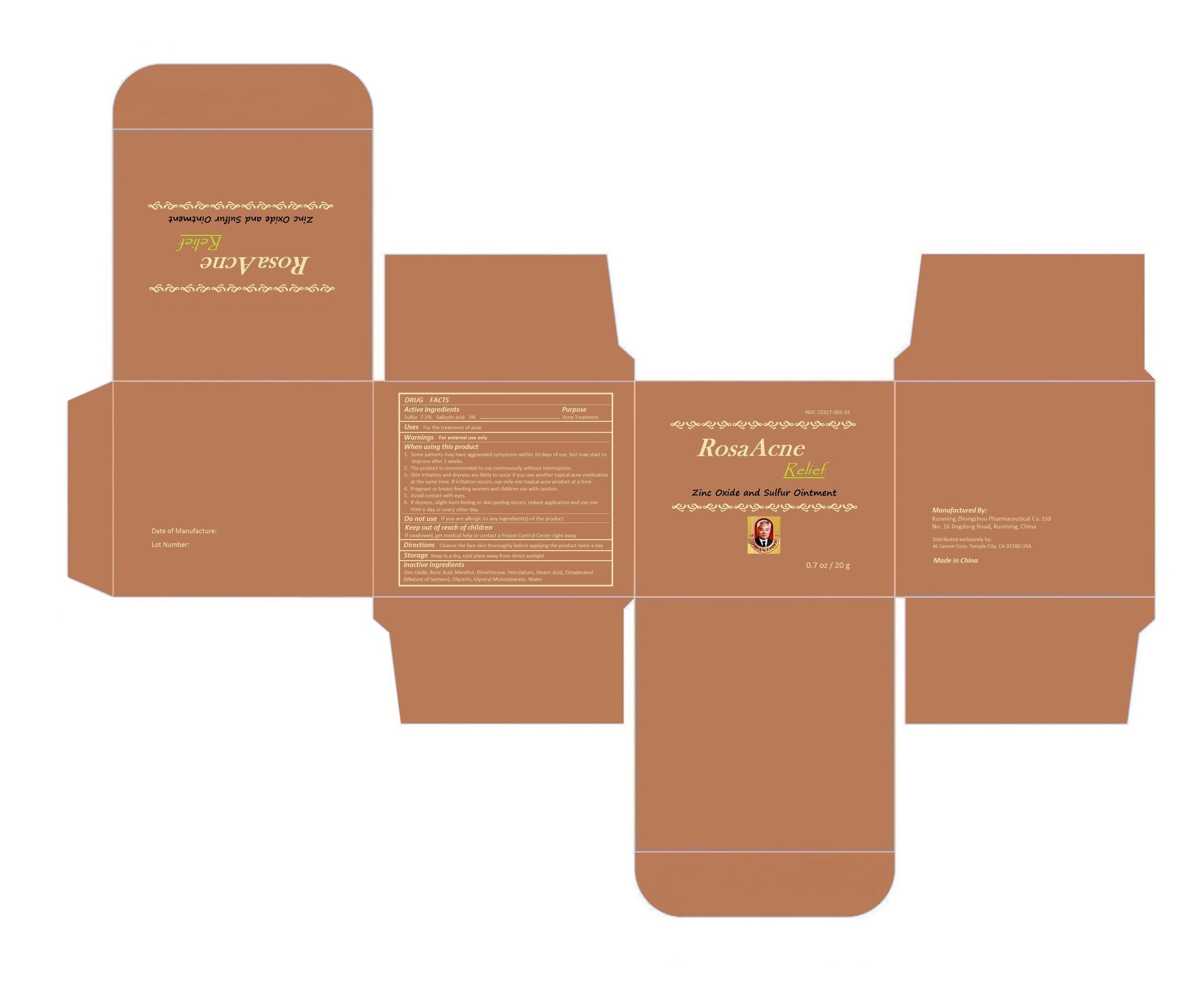
When using this product
1. Some patients may have aggravated symptoms within 10 days of use; but may start to improve after 2 weeks.
2. The product is recommended to use continuously without interruption.
3. Skin irritation and dryness are likely to occur if you use another topical acne medication at the same time. If irritation occurs, use only one topical acne product at a time.
4. Pregnant or breast-feeding women and children use with caution.
5. Avoid contact with eyes.
6. If dryness, slight burn feeling or skin peeling occurs, reduce application and use one time a day or every other day.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Manufactured by:
Kunming Zhongzhou Pharmaceutical Co. Ltd
No. 16 Jingdong Road, Kunming, China
Made in China
Distributed exclusively by:
AL Lauren Corp. Temple City, CA 91780 USA