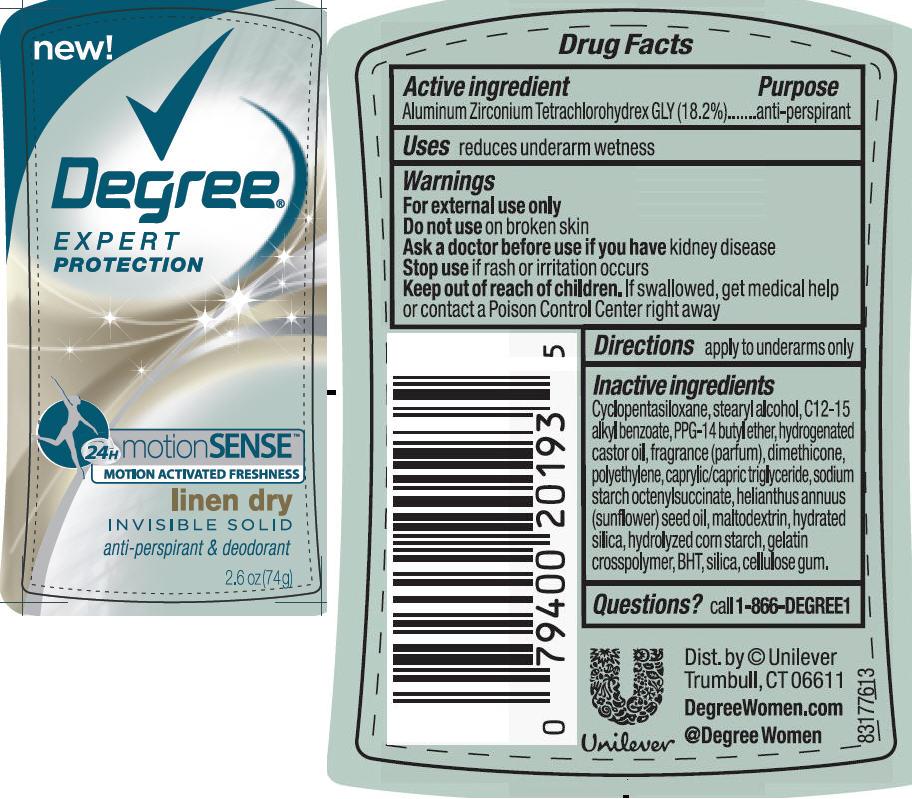
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away
Inactive ingredients
Cyclopentasiloxane
Stearyl Alcohol
C12-15 Alkyl Benzoate
PPG-14 Butyl Ether
Hydrogenated Castor Oil
Fragrance (Parfum)
Dimethicone
Polyethylene
Caprylic/Capric Triglyceride
Sodium Starch Octenylsuccinate
Helianthus Annuus (Sunflower) Seed Oil
Maltodextrin
Hydrated Silica
Hydrolyzed Corn Starch
Gelatin Crosspolymer
BHT
Silica
Cellulose Gum
Cyclopentasiloxane
Stearyl Alcohol
C12-15 Alkyl Benzoate
PPG-14 Butyl Ether
Hydrogenated Castor Oil
Fragrance (Parfum)
Dimethicone
Polyethylene
Caprylic/Capric Triglyceride
Sodium Starch Octenylsuccinate
Helianthus Annuus (Sunflower) Seed Oil
Maltodextrin
Hydrated Silica
Hydrolyzed Corn Starch
Gelatin Crosspolymer
BHT
Silica
Cellulose Gum