DESCRIPTION:
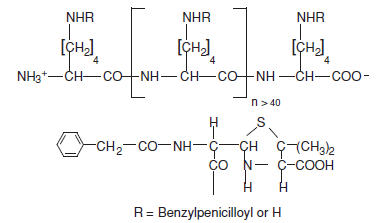
PRE-PEN® (benzylpenicilloyl polylysine) injection is a sterile solution of benzylpenicilloyl polylysine in a concentration of 6.0 X 10-5M (benzylpenicilloyl) in 0.01 M monobasic sodium phosphate and 0.15 M sodium chloride. Benzylpenicilloyl polylysine is a derivative of poly-l-lysine, where the epsilon amino groups are substituted with benzylpenicilloyl groups (50-70%) forming benzylpenicilloyl alpha amide. Each single-dose ampule contains 0.25 mL of PRE-PEN.
PRE-PEN has the following structure:
CLINICAL PHARMACOLOGY:
PRE-PEN is a skin test antigen reagent that reacts specifically with benzylpenicilloyl IgE antibodies initiating the release of chemical mediators which produce an immediate wheal and flare reaction at a skin test site. All individuals exhibiting a positive skin test to PRE-PEN possess IgE against the benzylpenicilloyl structural group which is a hapten. A hapten is a low molecular weight chemical that conjugates with a carrier (e.g. poly-l-lysine) resulting in the formation of an antigen with the hapten’s specificity. The benzylpenicilloyl hapten is the major antigenic determinant in penicillin-allergic individuals. However, many individuals reacting positively to PRE-PEN will not develop a systemic allergic reaction on subsequent exposure to therapeutic penicillin, especially among those who have not reacted to penicillins in the past. Thus, the PRE-PEN skin test determines the presence of penicilloyl IgE antibodies which are necessary but not sufficient for acute allergic reactions due to the major penicilloyl determinant.
Non-benzylpenicilloyl haptens are designated as minor determinants, since they less frequently elicit an immune response in penicillin treated individuals. The minor determinants may nevertheless be associated with significant clinical hypersensitivity. PRE-PEN does not react with IgE antibodies directed against non-benzylpenicilloyl haptens.
INDICATIONS AND USAGE:
PRE-PEN is indicated for the assessment of sensitization to penicillin (benzylpenicillin or penicillin G) in patients suspected to have clinical penicillin hypersensitivity. A negative skin test to PRE-PEN is associated with an incidence of immediate allergic reactions of less than 5% after the administration of therapeutic penicillin, whereas the incidence may be more than 50% in a history-positive patient with a positive skin test to PRE-PEN. These allergic reactions are predominantly dermatologic. Whether a negative skin test to PRE-PEN predicts a lower risk of anaphylaxis is not established. Similarly, when deciding the risk of proposed penicillin treatment, there are not enough data at present to permit relative weighing in individual cases of a history of clinical penicillin hypersensitivity as compared to positive skin tests to PRE-PEN and/or minor penicillin determinants.
CONTRAINDICATIONS:
PRE-PEN is contraindicated in those patients who have exhibited either a systemic or marked local reaction to its previous administration. Patients known to be extremely hypersensitive to penicillin should not be skin tested.
WARNINGS:
The risk of sensitization to repeated skin testing with PRE-PEN is not established. Rarely, a systemic allergic reaction including anaphylaxis (see below) may follow a skin test with PRE-PEN. To decrease the risk of a systemic allergic reaction, puncture skin testing should be performed first. Intradermal skin testing should be performed only if the puncture test is entirely negative.
PRECAUTIONS:
General:
No reagent, test, or combination of tests will completely assure that a reaction to penicillin therapy will not occur.
The value of the PRE-PEN skin test alone as a means of assessing the risk of administering therapeutic penicillin (when penicillin is the preferred drug of choice) in the following situations is not established:
- 1.
- Adult patients who give no history of clinical penicillin hypersensitivity.
- 2.
- Pediatric patients.
In addition, the clinical value of PRE-PEN where exposure to penicillin is suspected as a cause of a current drug reaction or in patients who are undergoing routine allergy evaluation is not known. Likewise, the clinical value of PRE-PEN skin tests alone in determining the risk of administering semi-synthetic penicillins (phenoxymethyl penicillin, ampicillin, carbenicillin, dicloxacillin, methicillin, nafcillin, oxacillin, amoxicillin), cephalosporin-derived antibiotics, and penem antibiotics is not known.
In addition to the results of the PRE-PEN skin test, the decision to administer or not administer penicillin should take into account individual patient factors. Healthcare professionals should keep in mind the following:
- 1.
- A serious allergic reaction to therapeutic penicillin may occur in a patient with a negative skin test to PRE-PEN.
- 2.
- It is possible for a patient to have an anaphylactic reaction to therapeutic penicillin in the presence of a negative PRE-PEN skin test and a negative history of clinical penicillin hypersensitivity.
- 3.
- If penicillin is the drug of choice for a life-threatening infection, successful desensitization with therapeutic penicillin may be possible irrespective of a positive skin test and/or a positive history of clinical penicillin hypersensitivity.
Pregnancy:
Animal reproduction studies have not been conducted with PRE-PEN. It is not known whether PRE-PEN can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. The hazards of skin testing in such patients should be weighed against the hazard of penicillin therapy without skin testing.
ADVERSE REACTIONS:
Occasionally, patients may develop an intense local inflammatory response at the skin test site. Rarely, patients will develop a systemic allergic reaction, manifested by generalized erythema, pruritus, angioedema, urticaria, dyspnea, hypotension, and anaphylaxis. The usual methods of treating a skin test antigen-induced reaction — the applications of a venous occlusion tourniquet proximal to the skin test site and administration of epinephrine are recommended. The patient should be kept under observation for several hours.
DOSAGE AND ADMINISTRATION:
SKIN TESTING DOSAGE AND TECHNIQUE
Skin testing responses can be attenuated by interfering drugs (e.g. H1-antihistamines and vasopressors). Skin testing should be delayed until the effects of such drugs have dissipated, or a separate skin test with histamine can be used to evaluate persistent antihistaminic effects in vivo. Due to the risk of potential systemic allergic reactions, skin testing should be performed in an appropriate healthcare setting under direct medical supervision.
Puncture Testing:
Skin testing is usually performed on the inner volar aspect of the forearm. The skin test antigen should always be applied first by the puncture technique. After preparing the skin surface, apply a small drop of PRE-PEN solution using a sterile 22-28 gauge needle. The same needle can then be used to make a single shallow puncture of the epidermis through the drop of PRE-PEN. Very little pressure is required to break the epidermal continuity. Observe for the appearance of a wheal, erythema, and the occurrence of itching at the test site during the succeeding 15 minutes at which time the solution over the puncture site is wiped off. A positive reaction consists of the development within 10 minutes of a pale wheal, sometimes with pseudopods, surrounding the puncture site and varying in diameter from 5 to 15 mm (or more). This wheal may be surrounded by a variable diameter of erythema, and accompanied by a variable degree of itching. The most sensitive individuals develop itching quickly, and the wheal and erythema are prompt in their appearance. As soon as a positive response as defined above is clearly evident, the solution over the scratch should be immediately wiped off. If the puncture test is either negative or equivocally positive (less than 5 mm wheal with little or no erythema and no itching), an intradermal test may be performed.
The lntradermal Test:
Using a 0.5 to 1.0 cc syringe with a 3/8” to 5/8”long, 26 to 30 gauge, short bevel needle, withdraw the contents of the ampule. Prepare with an alcohol swab a skin test area on the upper, outer arm, sufficiently below the deltoid muscle to permit proximal application of a tourniquet later, if necessary. Be sure to eject all air from the syringe through the needle, then insert the needle, bevel up immediately below the skin surface. Inject an amount of PRE-PEN sufficient to raise a small intradermal bleb of about 3 mm in diameter, in duplicate at least 2 cm apart. Using a separate syringe and needle, inject a like amount of saline or allergen diluting solution as a control at least 5 cm removed from the antigen test sites. Most skin reactions will develop within 5-15 minutes and response to the skin test is read at 20 minutes as follows:
Negative response — no increase in size of original bleb and no greater reaction than the control site.
Ambiguous response — wheal only slightly larger than initial injection bleb, with or without accompanying erythematous flare and slightly larger than the control site; OR discordance between duplicates.
Positive response — itching and significant increase in size of original blebs to at least 5 mm. Wheal may exceed 20 mm in diameter and exhibit pseudopods.
If the control site exhibits a wheal greater than 2-3 mm, repeat the test, and if the same reaction is observed, a physician experienced with allergy skin testing should be consulted.
HOW SUPPLIED: NDC 49471-001-05
PRE-PEN® (benzylpenicilloyl polylysine) injection is a clear, colorless, sterile solution in a concentration of 6.0 X 10-5M (benzylpenicilloyl) supplied in ampules containing 0.25 mL.
Box of 5 single-dose ampules. Ampules are opened by snapping the neck of the ampule using two forefingers of each hand. Visually inspect for glass shards before use. Each ampule is for single patient use only. Discard any unused portion.
Store PRE-PEN refrigerated at 2°C to 8°C (36°F to 46°F). If removed from refrigerator, PRE-PEN should be kept at room temperature up to 25°C (77°F) and must be used within 24 hours. Discard any unused portion. As with all parenteral drug products, PRE-PEN should be inspected visually for particulate matter and discoloration prior to administration. Report presence of particulate matter or discoloration to Manufacturer.
Rx only
Manufactured by
AllerQuest LLC
10 Farmington Valley Drive, Suite 106, Plainville, CT 06062
US License No. 2265
Distributed by
ALK-Abelló, Inc.
35 Channel Drive, Port Washington, NY 11050
Printed in USA
PRPE399999 12/2022
©2022 ALK-Abelló, Inc. and AllerQuest LLC
PRINCIPAL DISPLAY PANEL - Carton Label
Part No. PRPE399999 NDC 49471-001-05
benzylpenicilloyl polylysine
Pre-Pen®
injection
6.0 x 10-5M (benzylpenicilloyl)
For intradermal or subcutaneous use
SKIN TEST ANTIGEN
5 x 0.25 mL single-dose ampules
Rx Only
Distributed by
ALK-ABELLÓ
Manufactured by AllerQuest, LLC
GTIN 00349471001056
Serial No. 100001
Lot XXXXXX
Exp XX/XX
Made in USA
© 2022 ALK-Abelló, Inc.
© 2022 AllerQuest, LLC
Dosage: See Prescribing information.
Each single-dose ampule contains 6 x 10-5 M of benzylpenicilloyl
in 0.25 mL solution and also contains 0.01 M monobasic
sodium phosphate and 0.15 M sodium chloride to adjust the
ph to 7.6. Each ampule contains sufficient amount for both
puncture and intradermal testing for a single patient only
Discard unused portion
No preservative
Store refrigerated at 2° to 8°C (36°F to 46°F).
PRE-PEN can be stored at room temperature up to
25°C (77°F) for 24 hours. Discard PRE-PEN after 24 hours.
Discard if particulate matter or discoloration present.
NDC 49471-001-05 Part No. PRPE399999
Manufactured by:
AllerQuest, LLC
Plainville, CT 06062
US License No. 2265
Product of USA
Distributed by:
ALK-Abelló, Inc.
Port Washington, NY 11050