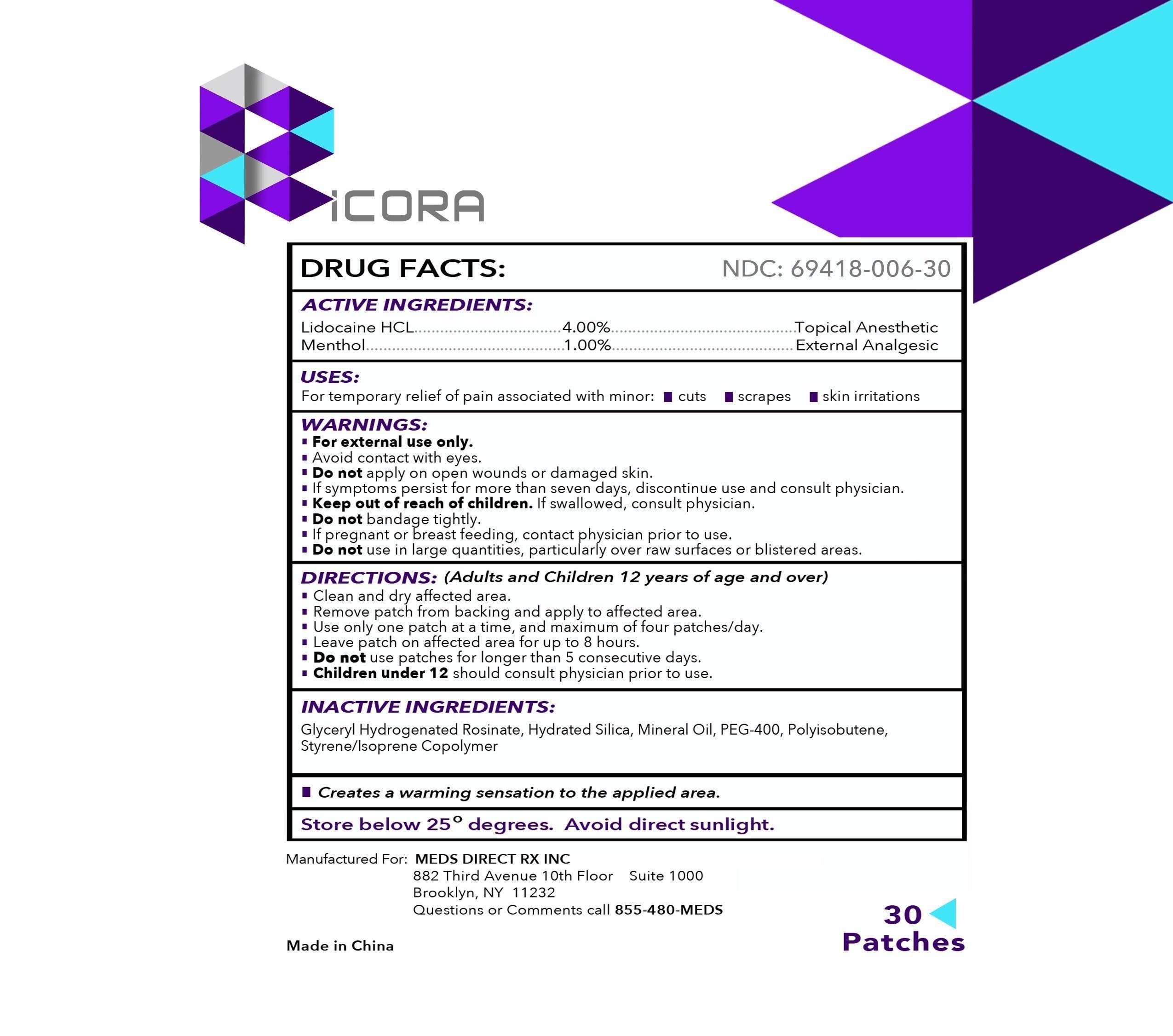
RICORA- lidocaine hydrochloride, menthol patch
Meds Direct Rx, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
ACTIVE INGREDIENTS:
Lidocaine HCL 4.00%
Menthol 1.00%
Topical Anesthetic
External Analgesic
USES:
For temporary relief of pain associated with minor: •cuts •scrapes •skin irritations
WARNINGS:
•
For external use only.
• Avoid contact with eyes.
•
Do not apply on open wounds or damaged skin.
• If symptoms persist for more than seven days, discontinue use and consult physician.
• Keep out of reach of children.
If swallowed, consult physician.
• Do not bandage tightly.
If pregnant or breastfeeding,
contact physician prior to use.
Do not
use in large quantities, particularly over raw surfaces or blistered areas.
DIRECTIONS:
(Adults and Children 12 years of age and over)
• Clean and dry affected area.
• Remove patch from backing and apply to affected area.
• Use only one patch at a time, and maximum of four patches/day.
• Leave patch on affected area for up to 8 hours.
•
Do not use patches for longer than 5 consecutive days.
•
Children under 12 should consult physician prior to use.
INACTIVE INGREDIENTS:
Glyceryl Hydrogenated Rosinate, Hydrated Silica, Mineral Oil, PEG-400, Polyisobutene, Styrene/Isoprene Copolymer
Creates a warming sensation to the applied area.
Store below 25 degrees. Avoid direct sunlight.
Package Lebeling:

Meds Direct Rx, Inc.

