FULL PRESCRIBING INFORMATION
1 Indications and Usage
KOĀTE® is a human plasma-derived antihemophilic factor indicated for the control and prevention of bleeding episodes or in order to perform emergency and elective surgery in patients with hemophilia A (hereditary Factor VIII deficiency).
Limitation of Use
KOĀTE is not indicated for the treatment of von Willebrand disease.
2 Dosage and Administration
For intravenous use after reconstitution only.
2.1 Dose
- Dose and duration of treatment depend on the severity of the Factor VIII deficiency, location and extent of bleeding, and the patient’s clinical condition.
- Each vial of KOĀTE is labeled with the actual Factor VIII potency in international units (IU). Calculation of the required dose of Factor VIII is based on the empirical finding that one IU of Factor VIII per kg body weight raises the plasma Factor VIII activity by approximately 2% of normal activity or 2 IU/dL.
- The required dose can be determined using the following formula:
Dose (IU) = Body Weight (kg) x Desired Factor VIII Rise (% normal or IU/dL) x 0.5
- Estimate the expected in vivo peak increase in Factor VIII level, expressed as IU/dL (or % normal), using the following formula:
Estimated Increment of Factor VIII
(% normal or IU/dL) = [Total Dose (IU)/Body Weight (kg)] x 2
- Patients may vary in their pharmacokinetic (e.g., half-life, in vivo recovery) and clinical responses. Base the dose and frequency on the individual clinical response.
Control and Prevention of Bleeding Episodes
A guide for dosing KOĀTE for the control and prevention of bleeding episodes (1,2) is provided in Table 1. Consideration should be given to maintaining a Factor VIII activity at or above the target range.
| Type of Bleeding | Factor VIII:C Level Required (% of normal) | Doses
(IU/kg) | Frequency of Doses (hours) | Duration of Therapy (days) |
| Minor Large bruises Significant cuts or scrapes Uncomplicated joint hemorrhage | 30 | 15 | 12 (twice daily) | Until hemorrhage stops and healing has been achieved (1–2 days). |
| Moderate Nose, mouth and gum bleeds Dental extractions Hematuria | 50 | 25 | 12 (twice daily) | Until healing has been achieved (2–7 days, on average). |
| Major
Joint hemorrhage Muscle hemorrhage Major trauma Hematuria Intracranial and intraperitoneal bleeding | 80-100 | Initial: 40-50 Maintenance: 25 | 12 (twice daily) | For at least 3–5 days Until healing has been achieved for up to 10 days. Intracranial hemorrhage may require prophylaxis therapy for up to 6 months. |
| Surgery | Prior to surgery: 80-100 After surgery: 60-100 | 40-50 30-50 | Once 12 (twice daily) | Prior to surgery For the next 7–10 days, or until healing has been achieved. |
2.2 Preparation and Reconstitution
- Use aseptic technique (clean and sanitized) and a flat work surface during the reconstitution procedure.
- Bring the vials of KOĀTE and the diluent (Sterile Water for Injection) to room temperature before use.
- Remove the shrink band from the KOĀTE vial. Do not use KOĀTE if the shrink band is absent or shows signs of tampering, and notify Grifols Therapeutics LLC immediately.
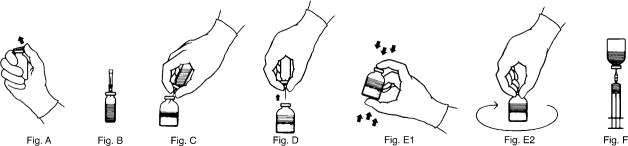
- Remove the plastic cap from the KOĀTE vial (Fig. A) and clean the top of the stopper with an alcohol swab. Allow the stopper to dry.
- Repeat this step with the vial of sterile water.
- Carefully remove the plastic sheath from the short end of the transfer needle and insert the exposed needle into the diluent vial to the hub (Fig. B)
- Place the KOĀTE vial upright on a flat surface. Remove the sheath from the other end of the transfer needle.
- While holding the KOĀTE vial securely on a flat surface insert the needle into the vial at a 45° angle to minimize foaming (Fig. C). The vacuum will draw the diluent into the concentrate vial. If vacuum is lost, use a sterile syringe and needle to remove the sterile water from the diluent vial and inject it into the KOĀTE, directing the stream of fluid against the wall of the vial.
- Remove the diluent vial and transfer needle (Fig. D).
- Agitate vigorously for 10-15 seconds, (Fig. E1) then swirl continuously until completely dissolved (Fig. E2). Avoid excessive foaming. The reconstituted solution should be clear to opalescent. Do not use if particulate matter and discoloration is observed.
- Clean the top of the vial of reconstituted KOĀTE with alcohol swab and let surface dry.
- Attach the filter needle (from the package) to a sterile syringe. Withdraw the KOĀTE solution into the syringe through the filter needle (Fig. F).
- Remove the filter needle from the syringe and discard the filter needle into a puncture proof container. Use KOĀTE within 3 hours after reconstitution. Do not refrigerate after reconstitution.
2.3 Administration
For intravenous administration only
- If the dose requires more than one vial of KOĀTE:
- Reconstitute each vial using a new transfer needle.
- Draw up all the solution into a single syringe.
- Visually inspect the final solution for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use if particulate matter or discoloration is observed.
- Attach the syringe to the connector end of an infusion set.
- Administer intravenously. The rate of administration should be determined by the patient’s comfort level, and no faster than 10 mL per minute.
3 DOSAGE FORMS AND STRENGTHS
KOĀTE is available as a lyophilized powder for reconstitution in single-use vials of 250, 500 and 1,000 IU of Factor VIII activity. The actual Factor VIII potency is labeled on each KOĀTE vial.
4 CONTRAINDICATIONS
KOĀTE is contraindicated in patients who have had hypersensitivity reactions, including anaphylaxis, to KOĀTE or its components.[see Description (11)]
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Hypersensitivity reactions, including anaphylaxis, are possible. Early signs of hypersensitivity reactions, which can progress to anaphylaxis, may include angioedema, chest tightness, hypotension, rash, nausea, vomiting, paresthesia, restlessness, wheezing and dyspnea. If hypersensitivity symptoms occur, discontinue use of the product immediately and administer appropriate emergency treatment.
5.2 Neutralizing Antibodies
The formation of neutralizing antibodies (inhibitors) to Factor VIII may occur. Monitor all patients for the development of Factor VIII inhibitors by appropriate clinical observations and laboratory tests. If expected plasma Factor VIII activity levels are not attained, or if bleeding is not controlled with an appropriate dose, perform an assay that measures Factor VIII inhibitor concentration. [see Warnings and Precautions (5.5)]
5.3 Intravascular Hemolysis
KOĀTE contains blood group isoagglutinins which are not clinically significant when small doses are used to treat minor bleeding episodes. However, when large and/or frequent doses of KOĀTE are given to patients with blood groups A, B, or AB, acute hemolytic anemia may occur, resulting in increased bleeding tendency or hyperfibrinogenemia. Monitor these patients for signs of intravascular hemolysis and falling hematocrit. [see Warnings and Precautions (5.5)] Should this condition occur, leading to progressive hemolytic anemia, discontinue KOĀTE and consider administering serologically compatible Type O red blood cells and providing alternative therapy.
5.4 Transmissible Infectious Agents
Because KOĀTE is made from human blood, it may carry a risk of transmitting infectious agents, e.g., viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent. There is also the possibility that unknown infectious agents may be present in the product. The risk that the product will transmit viruses has been reduced by screening plasma donors for prior exposure to certain viruses, by testing for the presence of certain current virus infections, and by inactivating and removing certain viruses during manufacture. Despite these measures, this product may still potentially transmit diseases.
Report all infections suspected by a physician possibly to have been transmitted by this product to Grifols Therapeutics LLC at 1-800-520-2807.
5.5 Monitoring: Laboratory Tests
- Monitor plasma Factor VIII activity levels by performing a validated test (e.g., one-stage clotting assay) to confirm that adequate Factor VIII levels have been achieved and maintained. [see Dosage ad Administration (2.1)]
- Monitor for the development of Factor VIII inhibitors. Perform a Bethesda inhibitor assay if expected Factor VIII plasma levels are not attained, or if bleeding is not controlled with the expected dose of KOĀTE. Use Bethesda Units (BU) to report inhibitor levels.
- Monitor for intravascular hemolysis and decreasing hematocrit values in patients with A, B or AB blood groups who are receiving large or frequent doses of KOĀTE.
6 ADVERSE REACTIONS
The most common adverse drug reactions (frequency ≥ 5 % of subjects) observed in the clinical trial were nervousness, headache, abdominal pain, nausea, paresthesia and blurred vision.
6.1 Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed cannot be directly compared to rates in other clinical trials and may not reflect the rates observed in practice.
The safety assessment of KOĀTE is based on data from a 2-stage, safety, pharmacokinetic (PK) and efficacy clinical trial in which twenty subjects with severe hemophilia A (<1% endogenous Factor VIII activity) were evaluable for safety. Nineteen subjects were enrolled in Stage I of the trial, including 15 Caucasian, 3 Hispanic, and 1 Black subjects. The mean age was 29 years (range: 13.9 – 46.4 years). Nineteen subjects, including the 18 subjects who completed Stage I, and one new subject were enrolled in Stage II. The mean age was 30 years (range: 13.9 – 46.4). The subjects received a total of 1053 infusions. Ten adverse reactions related to 7 infusions were reported in 4 subjects. These were: nervousness (2 subjects [10%]), headache (1 subject [5%]), abdominal pain (1 subject [5%]), nausea (1 subject [5%]), paresthesia (1 subject [5%]), and blurred vision (1 subject [5%]).
Immunogenicity
Subjects were monitored for neutralizing antibodies (inhibitors) to Factor VIII by the Bethesda assay at baseline and at 8, 17 and 26 weeks. No evidence of inhibitor formation was observed in the clinical trial.
The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, it may be misleading to compare the incidence of antibodies to KOĀTE in the study described above with the incidence of antibodies in other studies or to other products.
6.2 Postmarketing Experience
Because postmarketing reporting of adverse reactions is voluntary and from a population of uncertain size, it is not always possible to reliably estimate the frequency of these reactions or establish a causal relationship to product exposure.
- Blood and Lymphatic System Disorders: Factor VIII inhibition, hemolytic anemia
- Immune System Disorders: Hypersensitivity including anaphylaxis, rash, pruritus
- Injury, Poisoning and Procedural Complications: Post-procedural hemorrhage
- Nervous System Disorders: Generalized clonic-tonic seizure
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no data with KOĀTE use in pregnant women to inform on drug-associated risk. Animal reproduction studies have not been conducted using KOĀTE. It is not known whether KOĀTE can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. KOĀTE should be given to a pregnant woman only if clearly needed. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
8.2 Lactation
Risk Summary
There is no information regarding the presence of KOĀTE in human milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for KOĀTE and any potential adverse effects on the breast-fed infant from KOĀTE or from the underlying maternal condition.
8.4 Pediatric Use
Safety and efficacy studies have been performed in 20 previously treated pediatric patients aged 2.5 to 16 years. Subjects received 208 infusions of KOĀTE for treatment or control of bleeding episodes, including perioperative management, and routine prophylaxis. Children have shorter half-life and lower recovery of Factor VIII than adults. Because clearance of Factor VIII (based on per kilogram body weight) is higher in children, higher or more frequent dosing may be needed.
11 DESCRIPTION
KOĀTE, Antihemophilic Factor (Human), is a sterile, stable, dried concentrate of human antihemophilic factor in lyophilized powder form for reconstitution for intravenous injection. The product is supplied in single-use vials containing nominally 250, 500, or 1,000 international units (IU or units). Each vial of KOĀTE is labeled with the actual amount of Factor VIII expressed in IU. One IU is defined by the current World Health Organization International Standard for Factor VIII concentrate, which can be traced to the level of Factor VIII found in 1 mL of fresh pooled human plasma. The final product when reconstituted as directed contains not more than (NMT) 1500 μg/mL polyethylene glycol (PEG), NMT 0.05 M glycine, NMT 25 μg/mL polysorbate 80, NMT 5 μg/g tri-n-butyl phosphate (TNBP), NMT 3 mM calcium, NMT 1 μg/mL aluminum, NMT 0.06 M histidine, and NMT 10 mg/mL human albumin.
KOĀTE is purified from the cold insoluble fraction of pooled human plasma; the manufacturing process includes solvent/detergent (TNBP and polysorbate 80) treatment and heat treatment of the lyophilized final container. A gel permeation chromatography step serves the dual purpose of reducing the amount of TNBP and polysorbate 80 as well as increasing the purity of the Factor VIII in KOĀTE to 300 to 1,000 times over whole plasma. When reconstituted as directed, KOĀTE contains approximately 50 to 150 times as much Factor VIII as an equal volume of fresh plasma. The specific activity after addition of human albumin is in the range of 9 to 22 units/mg protein. KOĀTE also contains naturally occurring von Willebrand factor, which is co-purified as part of the manufacturing process.
The KOĀTE manufacturing process includes two dedicated steps with virus inactivation capacity. The solvent/detergent treatment step has the capacity to inactivate enveloped viruses (such as HIV, HCV, HBV, and WNV). Heat treatment at 80ºC for 72 hours has the capacity to inactivate enveloped viruses (such as HIV and HCV) as well as non‑enveloped viruses (such as HAV and B19V). The polyethylene glycol (PEG) precipitation/depth filtration step has the capacity to remove both enveloped and non‑enveloped viruses. The accumulated virus reduction factors for KOĀTE manufacturing process are presented in Table 2.
| * WNV inactivation was evaluated only for the solvent/detergent treatment step | ||||||||
|
Enveloped Viruses |
Non-enveloped Viruses |
|||||||
|
HIV-1 |
BVDV |
PRV |
VSV |
WNV |
Reo3 |
HAV |
PPV |
|
|
Model for |
HIV-1/2 |
HCV |
Large enveloped DNA viruses (e.g., herpes virus) |
Enveloped RNA viruses |
WNV |
Non-enveloped viruses |
HAV |
B19V |
|
Global Reduction Factor |
≥ 12.0 |
≥ 11.5 |
≥ 10.8 |
≥ 10.9 |
≥ 5.9* |
≥ 9.9 |
≥ 5.5 |
4.8 |
Additionally, the manufacturing process was investigated for its capacity to decrease the infectivity of an experimental agent of transmissible spongiform encephalopathy (TSE), considered a model for the variant Creutzfeldt-Jakob disease (vCJD) and Creutzfeldt-Jakob disease (CJD) agents. The manufacturing process has been shown to decrease TSE infectivity of that experimental model agent (a total of 5.1 log10 reduction), providing reasonable assurance that low levels of vCJD/CJD agent infectivity, if present in the starting material, would be removed.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
KOĀTE temporarily replaces the missing clotting Factor VIII that is needed for effective hemostasis.
12.2 Pharmacodynamics
Hemophilia A is a bleeding disorder characterized by a deficiency of functional coagulation Factor VIII, resulting in a prolonged plasma clotting time as measured by the activated partial thromboplastin time (aPTT) assay. Treatment with KOĀTE normalizes the aPTT over the effective dosing period.
12.3 Pharmacokinetics
The pharmacokinetics (PK) of KOĀTE were evaluated in a prospective, two-stage clinical trial of 20 previously treated patients (PTPs) with severe hemophilia A. In Stage I, the PK parameters for 19 subjects were based on plasma Factor VIII activity after a single intravenous infusion of 50 IU/kg of KOĀTE. Bioequivalence of the dry heat-treated KOĀTE to the unheated KOĀTE was demonstrated by comparison of Cmax and the area under the curve, AUC0-48 (Table 3). The incremental in vivo recovery ten minutes after infusion of dry heat-treated KOĀTE was 1.90% unit/kg (unheated KOĀTE was 1.82% units/kg). Mean biologic half-life was 16.1 hours.
In Stage II of the study, participants received KOĀTE treatments for six months on home therapy with a median of 52 days (range 23 to 94 days). At the end of 6 months, the mean AUC0-48 was 1471 ± 237 unit*hour/100 mL, the Cmax was 99 ± 13 unit/100 mL, and the t1/2was 16 ± 3.9 hours.
| Parameter | KOĀTE Dry Heat-treated (mean ± SD) | KOĀTE Unheated (mean ± SD) |
| AUC0-48(IU hr/mL)) | 1432 ±288 | 1477 ± 343 |
| Cmax(IU/mL) | 103 ± 19 | 99 ± 20 |
| Tmax(hr) | 0.41 ± 0.26 | 0.43 ± 0.44 |
| Half life (hr) | 16.1 ± 3.2 | 16.1 ± 5.1 |
14 CLINICAL STUDIES
The efficacy of KOĀTE for the treatment of bleeding episodes was demonstrated in a 2-stage, safety, PK and efficacy clinical trial. Stage I was a randomized, single-blind, single-dose, crossover, and PK study comparing heat-treated KOĀTE with unheated KOĀTE. Nineteen subjects were randomized and received a single dose of 50 IU/kg of either heated KOĀTE or unheated KOĀTE for PK assessment. Stage II was a 6 month open-label safety study conducted at two hemophilia centers. Nineteen subjects received KOĀTE, including for on-demand treatment and control of bleeding episodes. The study populations included 15 Caucasians, 3 Hispanic, and 1 Black subject. A total of 306 bleeding episodes were treated, of which 82% were treated with a single infusion of Factor VIII.
15 REFERENCES
-
Srivastava A, Brewer AK, Mauser-Bunschoten EP, et al. Guidelines for the management of hemophilia. Haemophilia 2013;19(1):e1-47.
- Abildgaard CF. Current concepts in the management of hemophilia. Semin Hematol 1975;12(3):223-32.
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
KOĀTE is supplied in single-use vials containing 250, 500 or 1,000 IU of Factor VIII activity, packaged with 5 mL or 10 mL of Sterile Water for Injection, one sterile double-ended transfer needle, one sterile filter needle, and one sterile administration set. The actual amount of KOĀTE in IU is stated on each carton and vial label.
Components used in the packaging of KOĀTE are not made with natural rubber latex.
|
Strength |
NDC Number |
|
250 IU |
76125-250-20, 76125-253-25 or |
|
500 IU |
76125-667-30, 76125-662-50 or |
|
1,000 IU |
76125-672-50, 76125-674-10 or |
Storage and Handling
- Store KOĀTE in its original package to protect it from light.
- Store the KOĀTE package at 2 to 8°C (36 to 46°F). Do not freeze.
- KOĀTE may also be stored at room temperature (up to 25°C or 77°F) for up to 6 months.
- Do not use after the expiration date.
- Use reconstituted KOĀTE immediately or within 3 hours of reconstitution.
17 PATIENT COUNSELING INFORMATION
- Inform patients to immediately report the following early signs and symptoms of hypersensitivity reactions to their healthcare professional: angioedema, chest tightness, hypotension, rash, nausea, vomiting, paresthesia, restlessness, wheezing and dyspnea. [see Warnings and Precautions (5.1)]
- Inform patients that the development of inhibitors to Factor VIII is a possible complication of treatment with KOĀTE. Advise the patients to contact their healthcare provider for further treatment and/or assessment if they experience a lack of clinical response to KOĀTE because this may be a manifestation of an inhibitor. [see Warnings and Precautions (5.2)]
- Inform patients that KOĀTE is made from human plasma and may carry a risk of transmitting infectious agents. While the risk that KOĀTE can transmit an infection has been reduced by screening plasma donors for prior exposure, testing donated plasma, and inactivating or removing certain viruses during manufacturing, patients should report any symptoms that concern them. [see Warnings and Precautions (5.4)
Manufactured for:
Kedrion Biopharma Inc.
400 Kelby Street
Fort Lee, NJ 07024
Manufactured by:
Grifols Therapeutics LLC
Research Triangle Park, NC 27709 USA
US License No. 1871
3058091
PACKAGE LABEL
NDC 76125-250-20
Koāte®
Antihemophilic Factor (Human)
250 IU FVIII Range
Solvent/Detergent Treated
Heat-Treated at 80°C
5 mL
Rx only
CONTENTS:
One bottle of Koāte
5 mL Sterile Water for Injection, USP
One sterile filter needle
One sterile double-ended transfer needle
One sterile administration set
No Preservative
For Intravenous Administration Only
Sterile — Nonpyrogenic
Date removed from refrigeration___________________
WARNING: THIS PRODUCT IS PREPARED FROM LARGE POOLS OF HUMAN PLASMA WHICH MAY CARRY THE RISK OF TRANSMITTING INFECTIOUS AGENTS.
The patient and physician should discuss the risks and benefits of this product.
Dosage and Administration: Read enclosed package insert.
Store refrigerated at 2 to 8°C (36 to 46°F) and no more than 6 months at room temperature (up to 25°C; 77°F) at any time prior to the expiration date. Avoid freezing.
Reconstitute with 5 mL Sterile Water for Injection, USP.
Administer within 3 hours after reconstitution.
This product when reconstituted contains not more than (NMT) 1500 μg/mL polyethylene glycol (PEG), NMT 0.05 M glycine, NMT 25 μg/mL polysorbate 80, NMT 5 μg/g tri-n-butyl phosphate (TNBP), NMT 3 mM calcium, NMT 1 μg/mL aluminum, NMT 0.06 M histidine, and NMT 10 mg/mL Albumin (Human).
If the shrink band is absent or shows any sign of tampering, do not use the product and notify Grifols Therapeutics LLC immediately.
Not Returnable for Credit or Exchange
Manufactured for:
Kedrion Biopharma Inc.
400 Kelby Street, Fort Lee, NJ 07024
Manufactured by:
Grifols Therapeutics LLC
Research Triangle Park, NC 27709 USA
U.S. License No. 1871
GTIN XXXXXXXXXXXXXX
LOT XXXXXXXXXX
EXP DDMMMYYYY
SN XXXXXXXXXXXXXXXX
IU XXX
Antihemophilic Factor (Human)
Carton: 3054099

NDC 76125-252-21
Koāte®
Antihemophilic Factor (Human)
Solvent/Detergent Treated
Heat-Treated at 80°C
Manufactured for:
Kedrion Biopharma Inc.
400 Kelby Street, Fort Lee, NJ 07024
Manufactured by:
Grifols Therapeutics LLC
Research Triangle Park, NC 27709 USA
U.S. License No. 1871
The patient and physician should discuss the risks and benefits of this product.
No Preservative
For Intravenous Administration Only
Sterile—Nonpyrogenic
Reconstitute with 5 mL Sterile Water for Injection, USP.
Store at 2–8°C (36–46°F) and no more than 6 months at room temperature (up to 25°C; 77°F) at any time prior to the expiration date.
Dosage and Administration: Read package insert.
Rx only
Date removed from refrigeration_____________________
Lot
Exp.
IU
3051798

NDC 76125-667-30
Koāte®
Antihemophilic Factor (Human)
500 IU FVIII Range
Solvent/Detergent Treated
Heat-Treated at 80°C
5 mL
Rx only
CONTENTS:
One bottle of Koāte
5 mL Sterile Water for Injection, USP
One sterile filter needle
One sterile double-ended transfer needle
One sterile administration set
No Preservative
For Intravenous Administration Only
Sterile — Nonpyrogenic
Date removed from refrigeration___________________
WARNING: THIS PRODUCT IS PREPARED FROM LARGE POOLS OF HUMAN PLASMA WHICH MAY CARRY THE RISK OF TRANSMITTING INFECTIOUS AGENTS.
The patient and physician should discuss the risks and benefits of this product.
Dosage and Administration: Read enclosed package insert.
Store refrigerated at 2 to 8°C (36 to 46°F) and no more than 6 months at room temperature (up to 25°C; 77°F) at any time prior to the expiration date. Avoid freezing.
Reconstitute with 5 mL Sterile Water for Injection, USP.
Administer within 3 hours after reconstitution.
This product when reconstituted contains not more than (NMT) 1500 μg/mL polyethylene glycol (PEG), NMT 0.05 M glycine, NMT 25 μg/mL polysorbate 80, NMT 5 μg/g tri-n-butyl phosphate (TNBP), NMT 3 mM calcium, NMT 1 μg/mL aluminum, NMT 0.06 M histidine, and NMT 10 mg/mL Albumin (Human).
If the shrink band is absent or shows any sign of tampering, do not use the product and notify Grifols Therapeutics LLC immediately.
Not Returnable for Credit or Exchange
Manufactured for:
Kedrion Biopharma Inc.
400 Kelby Street, Fort Lee, NJ 07024
Manufactured by:
Grifols Therapeutics LLC
Research Triangle Park, NC 27709 USA
U.S. License No. 1871
GTIN XXXXXXXXXXXXXX
LOT XXXXXXXXXX
EXP DDMMMYYYY
SN XXXXXXXXXXXXXXXX
IU XXX
Antihemophilic
Factor (Human)
Carton: 3054100

NDC 76125-669-31
Koāte®
Antihemophilic Factor (Human)
Solvent/Detergent Treated
Heat-Treated at 80°C
Manufactured for:
Kedrion Biopharma Inc.
400 Kelby Street, Fort Lee, NJ 07024
Manufactured by:
Grifols Therapeutics LLC
Research Triangle Park, NC 27709 USA
U.S. License No. 1871
The patient and physician should discuss the risks and benefits of this product.
No Preservative
For Intravenous Administration Only
Sterile—Nonpyrogenic
Reconstitute with 5 mL Sterile Water for Injection, USP.
Store at 2–8°C (36–46°F) and no more than 6 months at room temperature (up to 25°C; 77°F) at any time prior to the expiration date.
Dosage and Administration: Read package insert.
Rx only
Date removed from refrigeration_____________________
Lot
Exp.
IU
3051807

NDC 76125-672-50
Koāte®
Antihemophilic Factor (Human)
1000 IU FVIII Range
Solvent/Detergent Treated
Heat-Treated at 80°C
10 mL
Rx only
CONTENTS:
One bottle of Koāte
10 mL Sterile Water for Injection, USP
One sterile filter needle
One sterile double-ended transfer needle
One sterile administration set
No Preservative
For Intravenous Administration Only
Sterile — Nonpyrogenic
Date removed from refrigeration___________________
WARNING: THIS PRODUCT IS PREPARED FROM LARGE POOLS OF HUMAN PLASMA WHICH MAY CARRY THE RISK OF TRANSMITTING INFECTIOUS AGENTS.
The patient and physician should discuss the risks and benefits of this product.
Dosage and Administration: Read enclosed package insert.
Store refrigerated at 2 to 8°C (36 to 46°F) and no more than 6 months at room temperature (up to 25°C; 77°F) at any time prior to the expiration date. Avoid freezing.
Reconstitute with 10 mL Sterile Water for Injection, USP.
Administer within 3 hours after reconstitution.
This product when reconstituted contains not more than (NMT) 1500 μg/mL polyethylene glycol (PEG), NMT 0.05 M glycine, NMT 25 μg/mL polysorbate 80, NMT 5 μg/g tri-n-butyl phosphate (TNBP), NMT 3 mM calcium, NMT 1 μg/mL aluminum, NMT 0.06 M histidine, and NMT 10 mg/mL Albumin (Human).
If the shrink band is absent or shows any sign of tampering, do not use the product and notify Grifols Therapeutics LLC immediately.
Not Returnable for Credit or Exchange
Manufactured for:
Kedrion Biopharma Inc.
400 Kelby Street, Fort Lee, NJ 07024
Manufactured by:
Grifols Therapeutics LLC
Research Triangle Park, NC 27709 USA
U.S. License No. 1871
GTIN XXXXXXXXXXXXXX
LOT XXXXXXXXXX
EXP DDMMMYYYY
SN XXXXXXXXXXXXXXXX
IU XXXX
Antihemophilic
Factor (Human)
Carton: 3054101

NDC 76125-673-51
Koāte®
Antihemophilic Factor (Human)
Solvent/Detergent Treated
Heat-Treated at 80°C
Manufactured for:
Kedrion Biopharma Inc.
400 Kelby Street, Fort Lee, NJ 07024
Manufactured by:
Grifols Therapeutics LLC
Research Triangle Park, NC 27709 USA
U.S. License No. 1871
The patient and physician should discuss the risks and benefits of this product.
No Preservative
For Intravenous Administration Only
Sterile—Nonpyrogenic
Reconstitute with 10 mL Sterile Water for Injection, USP.
Store at 2–8°C (36–46°F) and no more than 6 months at room temperature (up to 25°C; 77°F) at any time prior to the expiration date.
Dosage and Administration: Read package insert.
Rx only
Date removed from refrigeration_____________________
Lot
Exp.
IU
3051812

NDC 13533-000-04
3053017
Nonpyrogenic
Single-Dose Container
5 mL
Sterile Water for Injection, USP
for reconstitution of accompanying product
Do not use unless clear. No antimicrobial agent or other substance has been added. Do not use for intravascular injection without making approximately isotonic by addition of suitable solute. Discard unused portion.
Rx Only.
Mfd by: Baxter Healthcare Corporation
Deerfield, IL 60015 USA
Mfd for: Grifols Therapeutics LLC
Research Triangle Park, NC 27709 USA
07-32-00-0008
Lot
Exp

NDC 76297-002-02
Sterile Water for Injection, USP
5 mL Rx Only
For reconstitution of accompanying product
Single-Dose Container, Nonpyrogenic
Do not use unless clear. No antimicrobial agent or other substance has been added. Do not use for intravascular injection without making approximately isotonic by addition of suitable solute. Discard unused portion.
Mfd by: Laboratorios Grifols, S.A. Parets del Vallès, Barcelona 08150 Spain
Lot
EXP
3057422

NDC 13533-200-05
Sterile Water for Injection, USP 5 mL Rx Only
For reconstitution of accompanying product
Single-Dose Container, Nonpyrogenic
Do not use unless clear. No antimicrobial agent or other
substance has been added. Do not use for intravascular
injection without making approximately isotonic by
addition of suitable solute. Discard unused portion.
Mfd by: Laboratorios Grifols, S. A., Parets del Vallès, Barcelona, 08150 Spain
Mfd for: Grifols Therapeutics LLC, Research Triangle Park, NC, 27709 USA
3051804
Lot / Exp.