FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
SUPREP Bowel Prep Kit is indicated for cleansing of the colon as a preparation for colonoscopy in adult and pediatric patients 12 years of age and older.
2 DOSAGE AND ADMINISTRATION
2.1 Dosage and Adminstration Overview
Administration of two bottles of SUPREP Bowel Prep Kit and additional water is required for a complete preparation for colonoscopy. One bottle of SUPREP Bowel Prep Kit is equivalent to one dose. SUPREP Bowel Prep Kit is supplied in two dosage strengths [see Dosage Forms and Strengths ( 3)] . The recommended dosage is:
•
Adults: Two 6-ounce doses
[see Dosage and Administration (
2.3)]
.
•
Pediatric patients 12 years of age and older: Two 4.5-ounce doses
[see Dosage and Administration (
2.4)]
.
2.2 Important Preparation and Administration Instructions
- Correct fluid and electrolyte abnormalities before treatment with SUPREP Bowel Prep Kit [see Warnings and Precautions ( 5.1)]
- Must dilute SUPREP Bowel Prep Kit in water before ingestion.
- Must consume additional water after each dose of SUPREP Bowel Prep Kit.
- On the day before colonoscopy, consume only a light breakfast or clear liquids (e.g., water, strained fruit juice without pulp, lemonade, plain coffee or tea, chicken broth, gelatin dessert without fruit). On the day of the colonoscopy only consume clear liquids up to two hours prior to colonoscopy.
- Do not eat solid food or drink milk or eat or drink anything colored red or purple.
- Do not drink alcohol.
- Do not take other laxatives while taking SUPREP Bowel Prep Kit.
- Do not take oral medications within one hour of starting each dose of SUPREP Bowel Prep Kit.
- If taking tetracycline or fluoroquinolone antibiotics, iron, digoxin, chlorpromazine, or penicillamine, take these medications at least 2 hours before and not less than 6 hours after administration of SUPREP Bowel Kit [see Drug Interactions ( 7.2)] .
- Stop consumption of all fluids at least 2 hours prior to the colonoscopy.
2.3 Recommended Dosage and Administration for Adults
The recommended Split-Dose (two-day) regimen for adults consists of two 6-ounce doses of SUPREP Bowel Prep Kit: the first dose during the evening prior to colonoscopy and the second dose the next day, during the morning of the colonoscopy.
Each dose consists of one bottle of SUPREP Bowel Prep Kit with additional water. The total volume of liquid required for colon cleansing (using two bottles) is 3 quarts. The following are recommended dosage and administration instructions for adults:
Dose 1 – On the Day Prior to Colonoscopy:
- May consume a light breakfast, or only clear liquids (no solid food).
- In the evening before the procedure, pour the contents of one bottle of SUPREP Bowel Prep Kit into the mixing container provided.
- Add cool drinking water to the 16-ounce fill line on the container, mix, and drink the entire amount.
- Drink two additional containers filled with water to the 16-ounce fill line over the next hour.
Dose 2 - Day of Colonoscopy:
- Continue to consume only clear liquids.
- In the morning (10 to 12 hours after the evening dose) on the day of the procedure, pour the contents of the second bottle of SUPREP Bowel Prep Kit into the mixing container provided.
- Add cool drinking water to the 16-ounce fill line on the container, mix, and drink the entire amount.
- Drink two additional containers filled with water to the 16-ounce fill line over the next hour.
- Complete all solution of SUPREP Bowel Prep Kit and required water at least two hours prior to colonoscopy.
2.4 Recommended Dosage and Administration for Pediatric Patients 12 Years of Age and Older
The recommended Split-Dose (two-day) regimen for pediatric patients 12 years of age and older consists of two 4.5-ounce doses of SUPREP Bowel Prep Kit: the first dose during the evening prior to colonoscopy and the second dose the next day, during the morning of the colonoscopy.
Each dose consists of one bottle of SUPREP Bowel Prep Kit with additional water. The total volume of liquid required for colon cleansing (using two bottles) is 2.25 quarts. The following are recommended dosage and administration instructions for pediatric patients 12 years of age and older and/or their caregivers:
Dose 1 – On the Day Prior to Colonoscopy:
• May consume a light breakfast, or only clear liquids (no solid food).
• In the evening before the procedure, pour the contents of one bottle of SUPREP Bowel Prep Kit into the mixing container provided.
• Add cool drinking water to the 12-ounce fill line on the container, mix, and drink the entire amount.
• Drink two additional containers filled with water to the 12-ounce fill line over the next hour.
Dose 2 – Day of Colonoscopy:
• Continue to consume only clear liquids.
• In the morning (10 to 12 hours after the evening dose) on the day of the procedure, pour the contents of the second bottle of SUPREP Bowel Prep Kit into the mixing container provided.
• Add cool drinking water to the 12-ounce fill line on the container, mix, and drink the entire amount.
• Drink two additional containers filled with water to the 12-ounce fill line over the next hour.
• Complete all solution of SUPREP Bowel Prep Kit solution and required water at least two hours prior to colonoscopy.
3 DOSAGE FORMS AND STRENGTHS
- SUPREP Bowel Prep Kit (for adults): Two bottles each containing 6 ounces of an oral solution of 17.5 grams sodium sulfate, 3.13 grams potassium sulfate, and 1.6 grams magnesium sulfate as a clear to slightly hazy liquid.
- SUPREP Bowel Prep Kit (for pediatric patients 12 years of age and older): Two bottles each containing 4.5 ounces of an oral solution of 13.13 grams sodium sulfate, 2.35 grams potassium sulfate, and 1.2 grams magnesium sulfate as a clear to slightly hazy liquid.
When diluted as directed, the solution is clear and colorless.
5 WARNINGS AND PRECAUTIONS
5.1 Serious Fluid and Serum Chemistry Abnormalities
Advise all patients to hydrate adequately before, during, and after the use of SUPREP Bowel Prep Kit. If a patient develops significant vomiting or signs of dehydration after taking SUPREP Bowel Prep Kit, consider performing post-colonoscopy lab tests (electrolytes, creatinine, and BUN).
Fluid and electrolyte disturbances can lead to serious adverse events including cardiac arrhythmias, seizures and renal impairment. Correct fluid and electrolyte abnormalities before treatment with SUPREP Bowel Prep Kit. Use SUPREP Bowel Prep Kit with caution in patients with conditions, or who are using medications, that increase the risk for fluid and electrolyte disturbances or may increase the risk of adverse events of seizure, arrhythmias, and renal impairment [see Drug Interactions ( 7.1)] .
SUPREP Bowel Prep Kit can cause temporary elevations in uric acid [see Adverse Reactions ( 6.1)] . Uric acid fluctuations in patients with gout may precipitate an acute flare. The potential for uric acid elevation should be considered before administering SUPREP Bowel Prep Kit to patients with gout or other disorders of uric acid metabolism.
5.2 Cardiac Arrhythmias
There have been rare reports of serious arrhythmias associated with the use of ionic osmotic laxative products for bowel preparation. Use caution when prescribing SUPREP Bowel Prep Kit for patients at increased risk of arrhythmias (e.g., patients with a history of prolonged QT, uncontrolled arrhythmias, recent myocardial infarction, unstable angina, congestive heart failure, or cardiomyopathy). Consider pre-dose and post-colonoscopy ECGs in patients at increased risk of serious cardiac arrhythmias.
5.3 Seizures
There have been reports of generalized tonic-clonic seizures and/or loss of consciousness associated with use of bowel preparation products in patients with no prior history of seizures. The seizure cases were associated with electrolyte abnormalities (e.g., hyponatremia, hypokalemia, hypocalcemia, and hypomagnesemia) and low serum osmolality. The neurologic abnormalities resolved with correction of fluid and electrolyte abnormalities.
Use caution when prescribing SUPREP Bowel Prep Kit for patients with a history of seizures and in patients at increased risk of seizure, such as patients taking medications that lower the seizure threshold (e.g., tricyclic antidepressants), patients withdrawing from alcohol or benzodiazepines, or patients with known or suspected hyponatremia [see Drug Interactions ( 7.1)] .
5.4 Use in Patients with Risk of Renal Injury
Use SUPREP Bowel Prep Kit with caution in patients with impaired renal function or patients taking concomitant medications that may affect renal function (such as diuretics, angiotensin converting enzyme inhibitors, angiotensin receptor blockers, or non-steroidal anti-inflammatory drugs) [see Drug Interactions ( 7.1)] . These patients may be at risk for renal injury. Advise these patients of the importance of adequate hydration with SUPREP Bowl Prep Kit and consider performing baseline and post-colonoscopy laboratory tests (electrolytes, creatinine, and BUN) in these patients [see Use in Specific Populations ( 8.6)] .
5.5 Colonic Mucosal Ulcerations and Ischemic Colitis
Osmotic laxative products may produce colonic mucosal aphthous ulcerations, and there have been reports of more serious cases of ischemic colitis requiring hospitalization. Concurrent use of stimulant laxatives and SUPREP Bowel Prep Kit may increase these risks [see Drug Interactions ( 7.3)] . Consider the potential for mucosal ulcerations resulting from the bowel preparation when interpreting colonoscopy findings in patients with known or suspect inflammatory bowel disease (IBD).
5.6 Use in Patients with Significant Gastrointestinal Disease
If gastrointestinal obstruction or perforation is suspected, perform appropriate diagnostic studies to rule out these conditions before administering SUPREP Bowel Prep Kit [see Contrandications ( 4)] .
Use with caution in patients with severe active ulcerative colitis.
6 ADVERSE REACTIONS
The following important adverse reactions for bowel preparations are described elsewhere in the labeling:
- Serious Fluid and Serum Chemistry Abnormalities [see Warnings and Precautions ( 5.1)]
- Cardiac Arrhythmias [see Warnings and Precautions ( 5.2)]
- Seizures [see Warnings and Precautions ( 5.3)]
- Use in Patients with Risk of Renal Injury [see Warnings and Precautions ( 5.4)]
- Colonic Mucosal Ulceration and Ischemic Colitis [see Warnings and Precautions ( 5.5)]
- Patients with Significant Gastrointestinal Disease [see Warnings and Precautions ( 5.6)]
- Aspiration [see Warnings and Precautions ( 5.7)]
6.1 Clinical Studies Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in clinical studies of another drug and may not reflect the rates observed in practice.
Adults
The safety of SUPREP Bowel Prep Kit was evaluated in a multi-center, randomized, active controlled trial in 379 adult patients undergoing colonoscopy [see Clinical Studies ( 14)] .
Most Common Adverse Reactions
Table 1 shows the most common adverse reactions reported in at least 2% of patients receiving SUPREP Bowel Prep Kit or the control (a bowel prep containing polyethylene glycol and electrolytes (PEG + E)) administered in split-dose (2-day) regimens.
|
* reported in at least 2% of patients |
||||
| Symptom | Split-Dose (2-Day) Regimen | |||
|
SUPREP %
|
PEG + E product %
|
|||
| Overall Discomfort | 54 | 67 | ||
| Abdominal Distension | 40 | 52 | ||
| Abdominal Pain | 36 | 43 | ||
| Nausea | 36 | 33 | ||
| Vomiting | 8 | 4 | ||
Laboratory Abnormalities
Table 2 shows the most common laboratory abnormalities (at least 10% in either treatment group and more than 2% difference between groups) for patients who developed new abnormalities of important electrolytes and uric acid after completing the bowel preparation with either SUPREP Bowel Prep Kit or PEG+E administered as a split-dose (2-day) regimen.
|
1 The study was not designed to support comparative claims for the laboratory abnormalities reported in this table. |
||||
|
2 Percent (n/N) of patients where N=number of patients with normal baseline who had abnormal values at the timepoint(s) of interest. |
||||
| Day of Colonoscopy
N (%) 2 | Day 30
N (%) 2 |
|||
| Bicarbonate (low) | SUPREP | 20 (13) | 7 (4) | |
| PEG + Electrolytes | 24 (15) | 4 (3) | ||
| Bilirubin, total (high) | SUPREP | 14 (9) | 0 (0) | |
| PEG + Electrolytes | 20 (12) | 3 (2) | ||
| BUN (high) | SUPREP | 2 (2) | 14 (11) | |
| PEG + Electrolytes | 4 (3) | 19 (15) | ||
| Calcium (high) | SUPREP | 16 (10) | 8 (5) | |
| PEG + Electrolytes | 6 (4) | 6 (4) | ||
| Chloride (high) | SUPREP | 4 (2) | 6 (4) | |
| PEG + Electrolytes | 20 (12) | 6 (4) | ||
| Osmolality (high) | SUPREP | 8 (6) | NA | |
| PEG + Electrolytes | 19 (13) | NA | ||
| Uric acid (high) | SUPREP | 27 (24) | 13 (12) | |
| PEG + Electrolytes | 12 (10) | 20 (17) | ||
Less Common Adverse Reactions
AV Block (1 case) and CK increase.
Adverse Reactions with Unapproved Use
In another study of 408 adult patients, higher rates of the following adverse reactions and laboratory abnormalities were reported in patients treated with SUPREP Bowel Prep Kit as an evening-only (1-day) regimen compared to the split-dose (2-day) regimen.
- overall discomfort, abdominal distention, nausea, and vomiting
- total bilirubin (high), BUN (high), creatinine (high), osmolality (high), potassium (high) and uric acid (high)
Administration of SUPREP Bowel Prep Kit in an evening-only (1-day) dosing regimen is not recommended.
Pediatrics 12 Years to 16 Years of Age
The safety of SUPREP Bowel Prep Kit was evaluated in a single dose-ranging clinical trial of 89 pediatric patients aged 12 years to 16 years [see Clinical Studies ( 14)] . In 26 pediatric patients who received SUPREP Bowel Prep Kit (two 4.5-ounce doses), the most common adverse reactions (> 10%) were nausea, abdominal pain, abdominal bloating, and vomiting.
7 DRUG INTERACTIONS
7.1 Drugs That May Increase Risks of Fluid and Electrolyte Abnormalities
Use caution when prescribing SUPREP Bowel Prep Kit to patients taking medications that increase the risk of fluid and electrolyte disturbances or may increase the risk of adverse events of seizure, arrhythmias, and prolonged QT in the setting of fluid and electrolyte abnormalities [see Warnings and Precautions ( 5.1, 5.2, 5.3, 5.4)].
7.2 Potential for Reduced Drug Absorption
SUPREP Bowel Prep Kit can reduce the absorption of other co-administered drugs [see Dosage and Administration ( 2.1)] .
- Administer oral medications at least one hour before starting each dose of SUPREP Bowel Prep Kit.
- Administer tetracycline and fluoroquinolone antibiotics, iron, digoxin, chlorpromazine, and penicillamine at least 2 hours before and not less than 6 hours after administration of SUPREP Bowel Prep Kit to avoid chelation with magnesium.
7.3 Stimulant Laxatives
Concurrent use of stimulant laxatives and SUPREP Bowel Prep Kit may increase the risk of mucosal ulceration or ischemic colitis. Avoid use of stimulant laxatives (e.g., bisacodyl, sodium picosulfate) while taking SUPRPEP Bowel Prep Kit [see Warnings and Precautions ( 5.5)] .
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on SUPREP Bowel Prep Kit use in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. Animal reproductive studies have not been conducted with sodium sulfate, potassium sulfate, and magnesium sulfate (SUPREP Bowel Prep Kit).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major bi rth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
8.2 Lactation
Risk Summary
There are no data available data on the presence of SUPREP Bowel Prep Kit in human or animal milk, the effects on the breastfed child, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for SUPREP Bowel Prep Kit and any potential adverse effects on the breastfed child from SUPREP Bowel Prep Kit or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of SUPREP Bowel Prep Kit (two 4.5-ounce doses) have been established for cleansing of the colon as a preparation for colonoscopy in pediatric patients 12 years of age and older. Use of SUPPREP Bowel Prep Kit in this age group is supported by evidence from an adequate and well-controlled trial of SUPREP Bowel Prep Kit in adults and a single, dose-ranging, controlled trial in 89 pediatric patients 12 years to 16 years of age [see Clinical Studies ( 14)] . In the pediatric trial, SUPREP Bowel Prep Kit (two 6-ounce doses) did not demonstrate additional treatment benefit and more patients reported gastrointestinal adverse reactions compared to SUPREP Bowel Prep Kit (two 4.5-ounce doses). Therefore, SUPREP Bowel Prep Kit (two 6-ounce doses) is not recommended for pediatric patients 12 years of age and older [see Dosage and Administration ( 2.3)] . The safety profile of SUPREP Bowel Prep Kit (two 4.5-ounce doses) in this pediatric population was similar to that seen in adults [see Adverse Reactions ( 6.1)] .
The safety and effectiveness of SUPREP Bowel Prep Kit in pediatric patients less than 12 years of age have not been established.
8.5 Geriatric Use
Of the 375 patients who received SUPREP Bowel Prep Kit in clinical trials, 94 (25%) were 65 years of age or older, and 25 (7%) were 75 years of age or older. No overall differences in safety or effectiveness of SUPREP Bowel Prep Kit, administered as the recommended split-dose (2-day) regimen, were observed between geriatric patients and younger patients. Geriatric patients reported more vomiting when SUPREP Bowel Prep Kit was given as a one-day preparation (not a recommended regimen). Elderly patients are more likely to have decreased hepatic, renal or cardiac function and may be more susceptible to adverse reactions resulting from fluid and electrolyte abnormalities [see Warnings and Precautions ( 5.1)] .
8.6 Renal Impairment
Use SUPREP Bowel Prep Kit with caution in patients with renal impairment or patients taking concomitant medications that may affect renal function. These patients may be at risk for renal injury. Advise these patients of the importance of adequate hydration before, during and after use of SUPREP Bowel Prep Kit and consider performing baseline and post-colonoscopy laboratory tests (electrolytes, creatinine, and BUN) in these patients [see Warnings and Precautions ( 5.4)] .
10 OVERDOSAGE
Overdosage of more than the recommended dose of SUPREP Bowel Prep Kit may lead to severe electrolyte disturbances, as well as dehydration and hypovolemia, with signs and symptoms of these disturbances. [see Warnings and Precautions ( 5.1, 5.2, 5.3) ]. Monitor for fluid and electrolyte disturbances and treat symptomatically.
11 DESCRIPTION
SUPREP Bowel Prep Kit (sodium sulfate, potassium sulfate, and magnesium sulfate) oral solution (for adults) is an osmotic laxative and is provided as two bottles each containing 6 ounces of solution. Each bottle contains: 17.5 grams sodium sulfate, 3.13 grams potassium sulfate, and 1.6 grams magnesium sulfate. Inactive ingredients include: citric acid USP, flavoring ingredients, malic acid FCC, sodium benzoate, NF, sucralose, purified water, USP.
SUPREP Bowel Prep Kit (sodium sulfate, potassium sulfate, and magnesium sulfate) oral solution (for pediatric patients 12 years of age and older) is an osmotic laxative and is provided as two bottles each containing 4.5 ounces of solution. Each bottle contains: 13.13 grams sodium sulfate, 2.35 grams potassium sulfate, and 1.2 grams magnesium sulfate. Inactive ingredients include: citric acid USP, flavoring ingredients, malic acid FCC, sodium benzoate, NF, sucralose, purified water, USP.
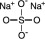
Sodium Sulfate, USP
The chemical name is Na 2SO 4. The average Molecular Weight is 142.04. The structural formula is:
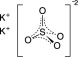
Potassium Sulfate, FCC, purified
The chemical name is K 2SO 4. The average Molecular Weight is 174.26. The structural formula is:
Magnesium Sulfate, USP
The chemical name is MgSO 4. The average Molecular Weight: 120.37. The structural formula is:
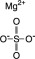
Each SUPREP Bowel Prep Kit also contains a polypropylene mixing container.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Sulfate salts provide sulfate anions, which are poorly absorbed. The osmotic effect of unabsorbed sulfate anions and the associated cations causes water to be retained within the gastrointestinal tract.
12.2 Pharmacodynamics
No formal pharmacodynamic studies have been conducted with SUPREP Bowel Prep Kit.
12.3 Pharmacokinetics
Absorption and Elimination
After administration of SUPREP Bowel Prep Kit in six healthy subjects, the time at which serum sulfate reached its highest point (Tmax) was approximately 17 hours after the first dose or approximately 5 hours after the second dose, and then declined with a half-life of 8.5 hours.
Excretion
Fecal excretion was the primary route of sulfate elimination.
Specific Populations
Patients with Renal Impairment
The disposition of sulfate after ingestion of SUPREP Bowel Prep Kit was studied in patients (N=6) with moderate renal impairment (creatinine clearance of 30 to 49 mL/min). In patients with moderate renal impairment, mean AUC was 54% higher and mean Cmax was 44% higher, than healthy subjects.
The mean sulfate concentrations in healthy subjects and in patients with moderate renal impairment returned to their respective baselines by Day 6 after dose initiation. Urinary excretion of sulfate over 30 hours after the first dose was approximately 16% lower in patients with moderate renal impairment than in healthy subjects. These differences are not considered clinically meaningful.
Patients with Hepatic Impairment
The disposition of sulfate after ingestion of SUPREP Bowel Prep Kit was studied in patients (N=6) with mild to moderate hepatic impairment (Child-Pugh grades A and B). Systemic exposure of serum sulfate (AUC and Cmax) was similar between healthy subjects and patients with hepatic impairment. The mean sulfate concentrations in healthy subjects and in patients with mild to moderate hepatic impairment returned
to their respective baselines by Day 6 after dose initiation. Urinary excretion of sulfate over 30 hours after the first dose was similar between patients with hepatic impairment and healthy subjects.
13 NONCLINICAL TOXICOLOGY
13.2 Animal Toxicology and/or Pharmacology
The sulfate salts of sodium, potassium, and magnesium contained in SUPREP Bowel Prep Kit were administered orally (gavage) to rats and dogs up to 28 days up to a maximum daily dose of 5 grams/kg/day (approximately 0.9 and 3 times for rats and dogs, respectively, the recommended human dose of 44 grams/day or 0.89 grams/kg based on the body surface area). In rats, the sulfate salts caused diarrhea and electrolyte and metabolic changes, including hypochloremia, hypokalemia, hyponatremia, lower serum osmolality, and high serum bicarbonate. Significant renal changes included increased fractional sodium excretion, increased urinary sodium and potassium excretion, and alkaline urine in both males and females. In addition, creatinine clearance was significantly decreased in females at the highest dose. No microscopic renal changes were seen. In dogs, the sulfate salts caused emesis, excessive salivation, excessive drinking of water, and abnormal excreta (soft and/or mucoid feces and/or diarrhea) and increased urine pH and sodium excretion.
14 CLINICAL STUDIES
Adults
The colon cleansing efficacy of SUPREP Bowel Prep Kit was evaluated in a randomized, single-blind, active-controlled, multicenter study in adult patients scheduled to have a colonoscopy. There were 363 adult patients included in the efficacy analysis. Patients ranged in age from 20 to 84 years (mean age 55 years) and 54% were female. Race distribution was 86% Caucasian, 9% African-American, and 5% other.
Patients were randomized to one of the following two colon preparation regimens: SUPREP Bowel Prep Kit or a marketed polyethylene glycol (PEG) plus electrolytes bowel preparation. In the Study SUPREP Bowel Prep Kit was administered as a split-dose (two-day) regimen. The PEG bowel prep was also given as a split-dose preparation according to its labeled instructions. Patients receiving SUPREP Bowel Prep Kit were limited to a light breakfast followed by clear liquids on the day prior to the day of colonoscopy; patients receiving the PEG bowel prep were allowed to have a normal breakfast and a light lunch, followed by clear liquids.
The primary efficacy endpoint was the proportion of patients with successful colon cleansing as assessed by the colonoscopists, who were not informed about the type of preparation received, as shown in Table 3. In the study, no clinically or statistically significant differences were seen between the group treated with SUPREP Bowel Prep Kit and the group treated with the PEG bowel prep.
|
1 Responders were patients whose colon preparations were graded excellent (no more than small bits of adherent feces/fluid) or good (small amounts of feces or fluid not interfering with the exam) by the colonoscopist. |
||||
|
2 Does not equal difference in tabled responder rates due to rounding effects. |
||||
| Treatment Group | Regimen | N | Responders1
% (95% C. I.) | SUPREP – PEG
Difference (95% CI) |
| SUPREP Bowel Prep Kit
(with light breakfast) | Split-Dose | 180 | 97%
(94%, 99%) |
2%2 (-2%, 5%) |
| PEG bowel prep
(with normal breakfast & light lunch) | Split-Dose | 183 | 96%
(92%, 98%) |
|
Pediatric Patients 12 Years to 16 Years of Age
SUPREP Bowel Prep Kit was evaluated for colon cleansing in a randomized, single-blind, multicenter, doseranging, active-controlled study in 89 pediatric patients 12 years to 16 years of age. The majority of patients were female (57%), white (78%), and of non-Hispanic or non-Latino ethnicity (91%). The mean age was 14 years. The median body weight was 60 kg (range 32 to 155 kg).
Patients were randomized to SUPREP Bowel Prep Kit (two 6-ounce doses), SUPREP Bowel Prep Kit (two 4.5-ounce doses) or oral PEG solution. SUPREP Bowel Prep Kit (two 6-ounce doses) did not demonstrate additional treatment benefit and more patients reported gastrointestinal adverse reactions compared to SUPREP Bowel Prep Kit (two 4.5-ounce doses); therefore, SUPREP Bowel Prep Kit (two 6-ounce doses) is not recommended for pediatric patients 12 years of age and older [see Dosage and Administration ( 2.4)] .
Patients in the SUPREP Bowel Prep Kit (two 4.5-ounce doses) group took the preparation in a “split-dose” regimen, where the first dose was taken the evening before colonoscopy, with the second dose taken the morning of the exam. Patients in the control group took the preparation according to its approved labeling on the evening before colonoscopy.
Patients in the SUPREP Bowel Prep Kit group (two 4.5-ounce doses) were allowed to have a light breakfast on the day before colonoscopy, followed by clear liquids until the colonoscopy is completed the following day. Patients in the control group subjects were permitted only clear liquids on the day prior to colonoscopy until completion of the colonoscopy the following day.
The primary efficacy endpoint was the proportion of patients with successful colon cleansing as assessed by the colonoscopists, who were not informed about the type of preparation received.
The percentage of responders and the associated 95% confidence intervals for the SUPREP Bowel Prep Kit (two 4.5-ounce doses) and Oral PEG solution are shown in Table 4. Efficacy was similar between patients who weighed 65 kg or more (n=12) and those patients who weighed less than 65 kg (n=15) in the SUPREP Bowel Prep Kit (two 4.5-ounce doses) arm.
|
1 Responders were patients whose colon preparations were graded excellent (no more than small bits of adherent feces/fluid) or good (small amounts of feces or fluid not interfering with the exam) by the colonoscopist. |
||||
|
2 Does not equal difference in tabled responder rates due to rounding effects. |
||||
| Treatment Group | Regimen | N | Responders1
% (95% C. I.) | SUPREP – PEG
Difference (95% CI) |
|
SUPREP Bowel Prep Kit 4.5 ounces per dose
| Split-Dose | 26 | 85%
(71%, 99%) |
25%2 (3%, 47%) |
| Oral PEG Solution
(with clear liquids only) |
Evening Dosing | 32 | 59%
(42%, 76%) |
|
16 HOW SUPPLIED/STORAGE AND HANDLING
Each SUPREP Bowel Prep Kit (sodium sulfate, potassium sulfate, and magnesium sulfate) oral solution (for adults) (NDC 52268-012-01) contains:
- Two bottles (NDC 52268-011-01) each containing 6-ounces of an oral solution of 17.5 grams sodium sulfate, 3.13 grams potassium sulfate, and 1.6 grams magnesium sulfate as a clear to slightly hazy liquid. When diluted as directed, the solution is clear and colorless.
- One (1) mixing container with a 16-ounce fill line.
Each SUPREP Bowel Prep Kit (sodium sulfate, potassium sulfate, and magnesium sulfate) oral solution (for pediatric patients 12 years of age and older) (NDC 52268-112-01) contains:
- Two bottles (NDC 52268-111-01) each containing 4.5-ounces of an oral solution of 13.13 grams sodium sulfate, 2.35 grams potassium sulfate, and 1.2 grams magnesium sulfate as a clear to slightly hazy liquid. When diluted as directed, the solution is clear and colorless.
- One (1) mixing container with a 12-ounce fill line.
17 PATIENT COUNSELING INFORMATION
Advise the patient and/or caregiver to read the FDA-approved patient labeling (Medication Guide).
Instruct patients or caregivers:
- Must dilute SUPREP Bowel Prep Kit before ingestion.
- Must consume additional water after each dose of SUPREP Bowel Prep Kit.
- On the day before colonoscopy, consume only a light breakfast or clear liquids (e.g., water, apple or orange juice without pulp, lemonade, coffee, tea, or chicken broth). On the day of the colonoscopy only consume clear liquids up to two hours prior to colonoscopy.
- Two doses of SUPREP Bowel Prep Kit are required for a complete preparation for colonoscopy. One bottle of SUPREP Bowel Prep Kit is equivalent to one dose.
- Do not to take other laxatives while taking SUPREP Bowel Prep Kit.
- Do not eat solid food or drink milk or eat or drink anything colored red or purple.
- Do not drink alcohol.
- Do not take oral medications within one hour of starting each dose of SUPREP Bowel Prep Kit.
- If taking tetracycline or fluoroquinolone antibiotics, iron, digoxin, chlorpromazine, or penicillamine, take these medications at least 2 hours before and not less than 6 hours after administration of SUPREP Bowel Prep Kit [see Drug Interactions ( 7.2)] .
- Stop consumption of all fluids at least 2 hours prior to colonoscopy.
- Contact their healthcare provider if they develop significant vomiting or signs of dehydration after taking SUPREP Bowel Prep Kit or if they experience cardiac arrhythmias or seizures [see Warnings and Precautions ( 5.1, 5.2, 5.3)] .
Distributed by Braintree Laboratories, Inc.
Braintree, MA 02185
U.S. Patent 6,946,149
|
MEDICATION GUIDE SUPREP ® (Soo-prep) Bowel Prep Kit (sodium sulfate, potassium sulfate and magnesium sulfate) oral solution |
| Read and understand this Medication Guide instructions at least 2 days before your colonoscopy and again before you start taking SUPREP Bowel Prep Kit. |
|
What is the most important information I should know about SUPREP Bowel Prep Kit? SUPREP Bowel Prep Kit and other bowel preparations can cause serious side effects, including:
Your chance of having fluid loss and changes in body salts with SUPREP Bowel Prep Kit is higher if you: • have heart problems• have kidney problems • take water pills or non-steroidal anti-inflammatory drugs (NSAIDS) Tell your healthcare provider right away if you have any of these symptoms of a loss of too much body fluid (dehydration) while taking SUPREP Bowel Prep Kit: o vomiting o urinating less often than normal
|
|
What is SUPREP Bowel Prep Kit? SUPREP Bowel Prep Kit is a prescription medicine used by adults and children 12 years of age and older to clean the colon before a colonoscopy. SUPREP Bowel Prep Kit cleans your colon by causing you to have diarrhea. Cleaning your colon helps your healthcare provider see the inside of your colon more clearly during your colonoscopy.
|
|
Do not take SUPREP Bowel Prep Kit if your healthcare provider has told you that you have:
|
|
Before taking SUPREP Bowel Prep Kit, tell your healthcare provider about all of your medical conditions, including if you: • have problems with serious loss of body fluid (dehydration) and changes in blood salts (electrolytes).
• are breastfeeding or plan to breastfeed. It is not known if SUPREP Bowel Prep Kit passes into your breast milk. You and your healthcare
Especially tell your healthcare provider if you take:
The following medicines should be taken at least 2 hours before starting SUPREP Bowel Prep Kit and not less than 6 hours after taking SUPREP Bowel Prep Kit:
Ask your healthcare provider or pharmacist for a list of these medicines if you are not sure if you are taking any of the medicines listed above. Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine. |
|
How should I take SUPREP Bowel Prep Kit? See the Instructions for Use in the Patient Instructions for Use Booklet for dosing instructions. You must read, understand, and follow these instructions to take SUPREP Bowel Prep Kit the right way.
• While taking SUPREP Bowel Prep Kit, do not: • take any other laxatives.
|
|
What are the possible side effects of SUPREP Bowel Prep Kit?
• See
What is the most important information I should know about SUPREP Bowel Prep Kit?
o overall discomfort o stomach bloating
These are not all the possible side effects of SUPREP Bowel Prep Kit. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|
How should I store SUPREP Bowel Prep Kit? • Store SUPREP Bowel Prep Kit at room temperature, between 68°F to 77°F (20°C to 25°C). Keep SUPREP Bowel Prep Kit and all medicines out of the reach of children. |
|
General information about the safe and effective use of SUPREP Bowel Prep Kit.
|
|
What are the ingredients in SUPREP Bowel Prep Kit? SUPREP Bowel Prep Kit is supplied in two dosage strengths. SUPREP Bowel Prep Kit comes in a carton containing two 6-ounce bottles, along with a 16-ounce polypropylene mixing container. SUPREP Pediatric Bowel Prep Kit comes in a carton containing two bottles containing 4.5 ounces of
Each bottle contains:
Distributed by Braintree Laboratories, Inc.
|
This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised 08/2020
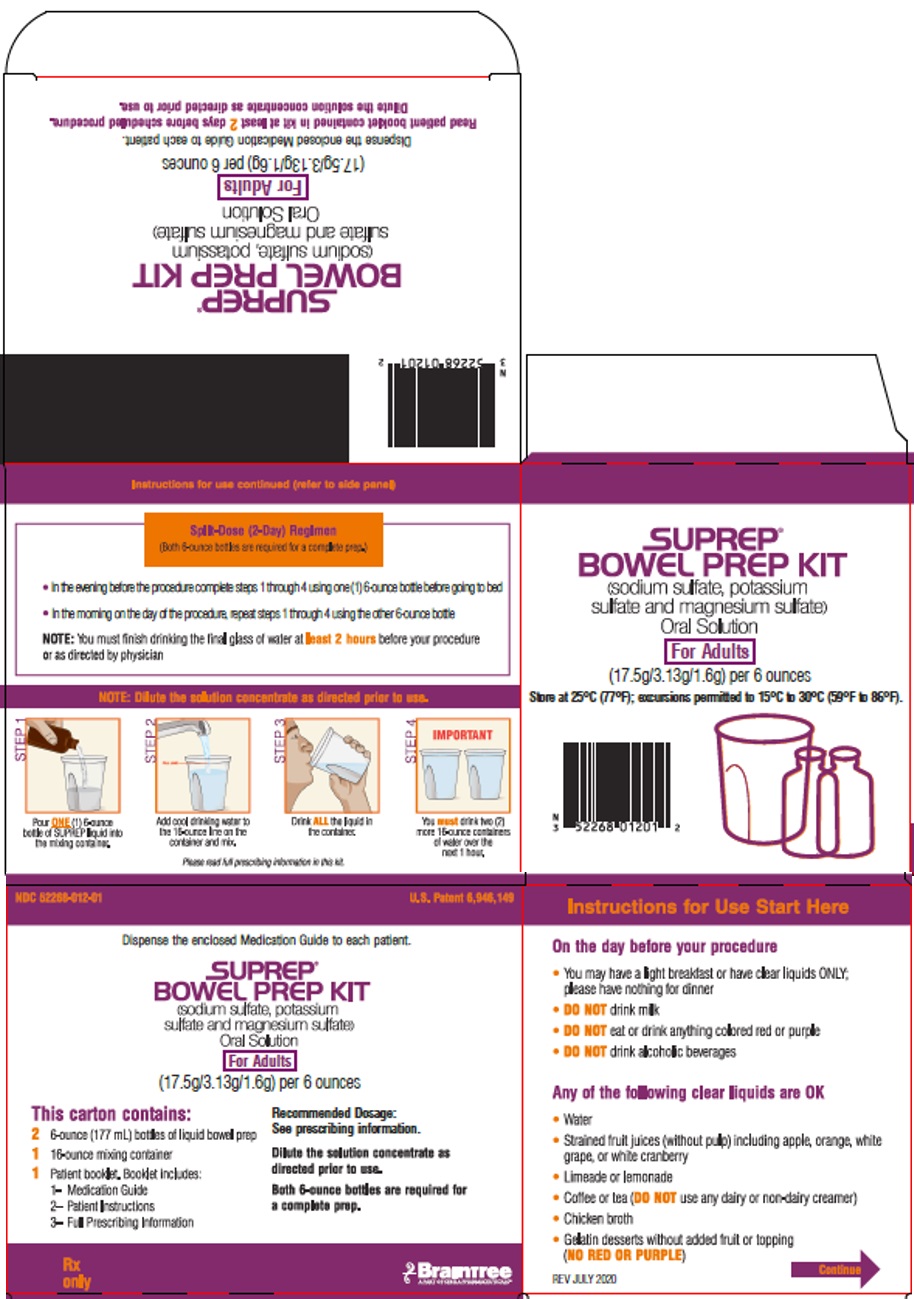
Principal Display Panel – Adult Carton Label
NDC 52268-012-01 U.S. Patent 6,946,149
Dispense the enclosed Medication Guide to each patient.
SUPREP
BOWEL PREP KIT
(sodium sulfate, potassium
sulfate and magnesium sulfate)
Oral Solution
For Adults
(17.5g/3.13g/1.6g) per 6 ounces
This carton contains:
2 6-ounce (177 mL) bottles of liquid bowel prep
1 16-ounce mixing container
1 Patient booklet. Booklet includes:
1- Medication Guide
2- Patient Instructions
3- Full Prescribing Information
Recommended Dosage:
See prescribing information
Dilute the solution concentrate as
directed prior to use.
Both 6-ounce bottles are required for
a complete prep.
Rx only
Braintree
A PART OF SEBELA PHARMACEUTICALS
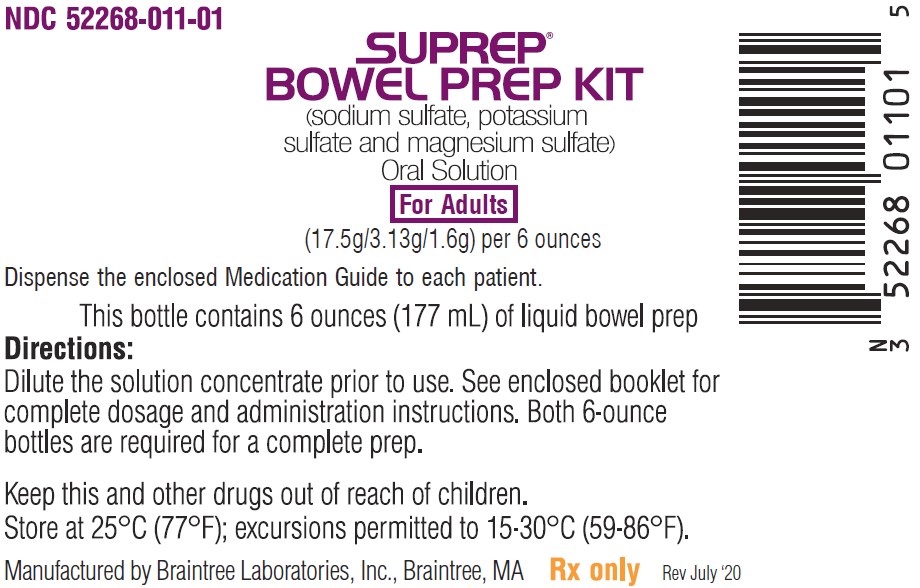
Principal Display Panel – Adult Bottle Label
NDC 52268-011-01
SUPREP
BOWEL PREP KIT
(sodium sulfate, potassium
sulfate and magnesium sulfate)
Oral Solution
For Adults
(17.5g/3.13g/1.6g) per 6 ounces
Dispense the enclosed Medication Guide to each patient.
This bottle contains 6 ounces (177 mL) of liquid bowel prep
Directions:
Dilute the solution concentrate prior to use. See enclosed booklet for
complete dosage and administration instructions. Both 6-ounce
bottles are required for a complete prep.
Keep this and other drugs out of reach of children.
Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F).
Manufactured by Braintree Laboratories, Braintree, MA Rx only Rev July ‘20
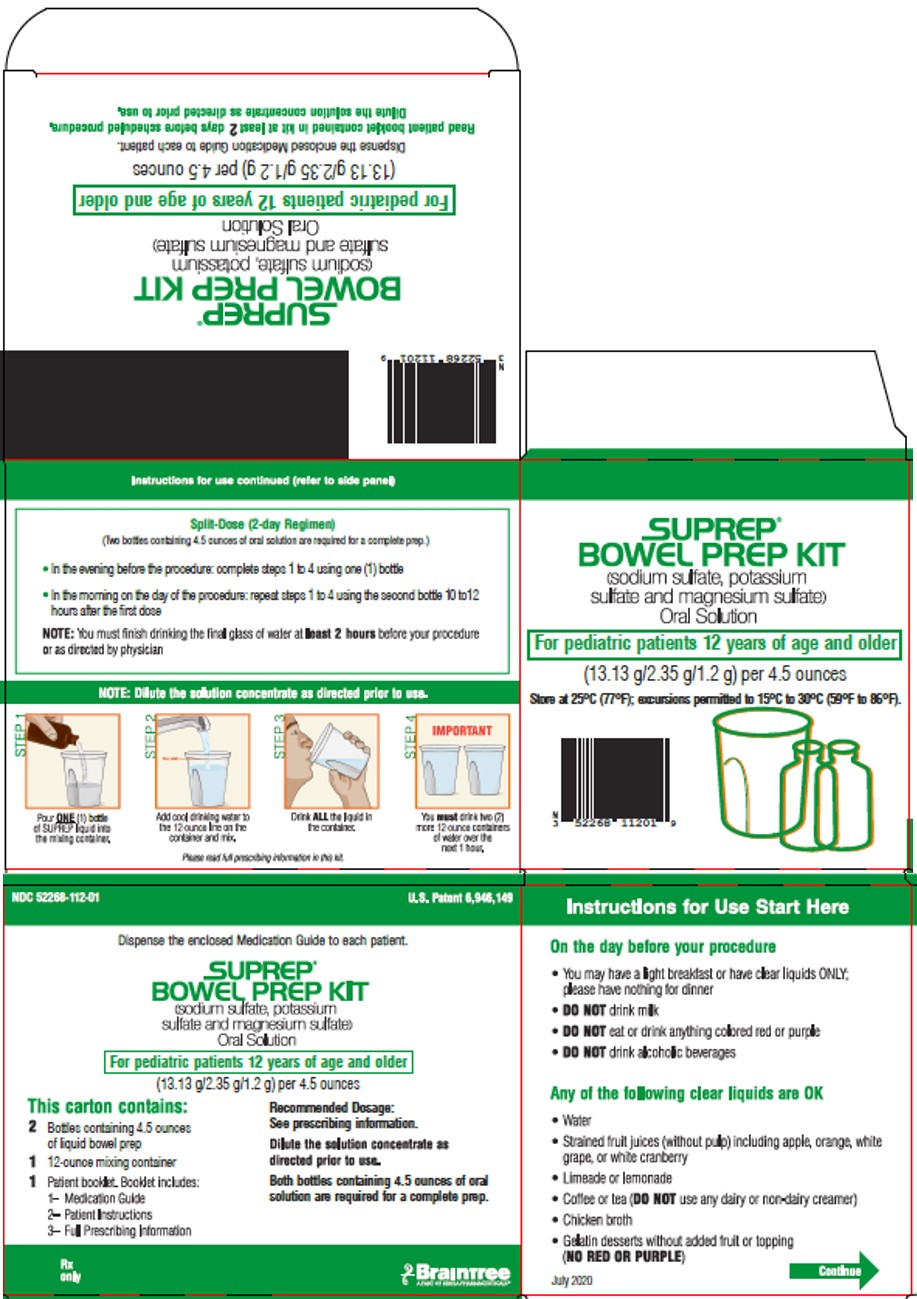
Principal Display Panel – Pediatric Carton Label
NDC 52268-112-01 U.S. Patent 6,946,149
Dispense the enclosed Medication Guide to each patient.
SUPREP®
BOWEL PREP KIT
(sodium sulfate, potassium
sulfate and magnesium sulfate)
Oral Solution
For pediatric patients 12 years of age and older
(13.13g/2.35g/1.2g) per 4.5 ounces
This carton contains:
2 Bottles containing 4.5 ounces
of liquid bowel prep
1 12-ounce mixing container
1 Patient booklet. Booklet includes:
1- Medication Guide
2- Patient Instructions
3- Full Prescribing Information
Recommended Dosage:
See prescribing information
Dilute the solution concentrate as
directed prior to use.
Both bottles containing 4.5 ounces of oral
solutions are required for a complete prep.
Rx only
Braintree
A PART OF SEBELA PHARMACEUTICALS
Principal Display Panel – Pediatric Bottle Label
NDC 52268-111-01
SUPREP®
BOWEL PREP KIT
(sodium sulfate, potassium
sulfate and magnesium sulfate)
Oral Solution
For pediatric patients 12 years of age and older
(13.13g/2.35g/1.2g) per 6 ounces
Dispense the enclosed Medication Guide to each patient.
This bottle contains 4.5 ounces of liquid bowel prep
Directions:
Dilute the solution concentrate prior to use. See enclosed booklet for
complete dosage and administration instructions. Both 6 bottles
containing 4.5 ounces of oral soultion are required for a complete prep.
Keep this and other drugs out of reach of children.
Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F).
Manufactured by Braintree Laboratories, Braintree, MA Rx only July 2020