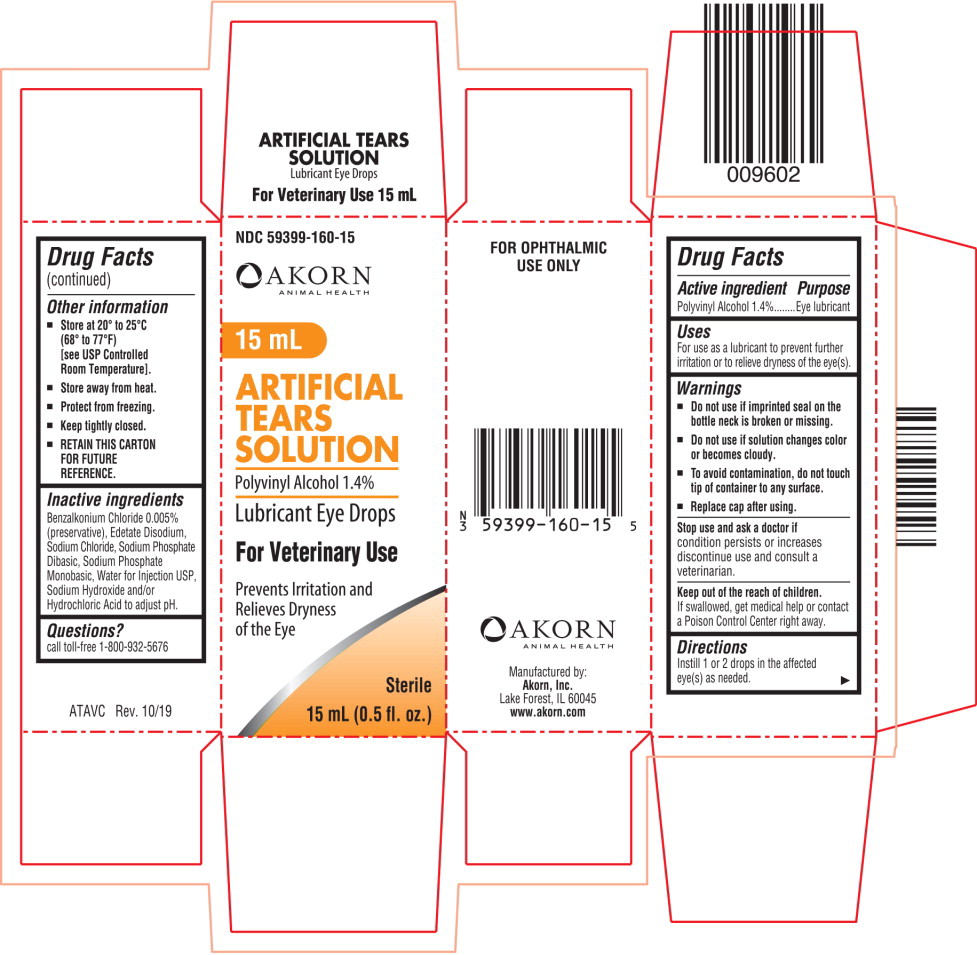
Active ingredient
Polyvinyl Alcohol 1.4%
Uses
For use as a lubricant to prevent further irritation or to relieve dryness of the eye(s).
Warnings
-
Do not use if imprinted seal on the bottle neck is broken or missing.
-
Do not use if solution changes color or becomes cloudy.
-
To avoid contamination, do not touch tip of container to any surface.
-
Replace cap after using.
Stop use and ask a doctor if
condition persists or increases discontinue use and consult a veterinarian.
Keep out of the reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Instill 1 or 2 drops in the affected eye(s) as needed.
Other information
-
Store at 20° to 25°C (68° to 77°F)
[see USP Controlled Room Temperature].
-
Store away from heat.
-
Protect from freezing.
-
Keep tightly closed.
-
RETAIN THIS CARTON FOR FUTURE REFERENCE.
Inactive ingredients
Benzalkonium Chloride 0.005% (preservative), Edetate Disodium, Sodium Chloride, Sodium Phosphate Dibasic, Sodium Phosphate Monobasic, Water for Injection USP, Sodium Hydroxide and/or Hydrochloric Acid to adjust pH.
Questions?
call toll-free 1-800-932-5676
Principal Display Panel Text for Container Label:
NDC 59399-160-15
15 mL
ARTIFICIAL
TEARS
SOLUTION
Polyvinyl Alcohol 1.4%
Lubricant Eye Drops
For Veterinary Use
Sterile
15 mL (0.5 fl. oz.)
Principal Display Panel Text for Carton Label:
NDC 59399-160-15
Akorn Animal Health Logo
15 mL
ARTIFICIAL
TEARS
SOLUTION
Polyvinyl Alcohol 1.4%
Lubricant Eye Drops
For Veterinary Use
Prevents Irritation and
Relieves Dryness
of the Eye
Sterile
15 mL (0.5 fl. oz.)