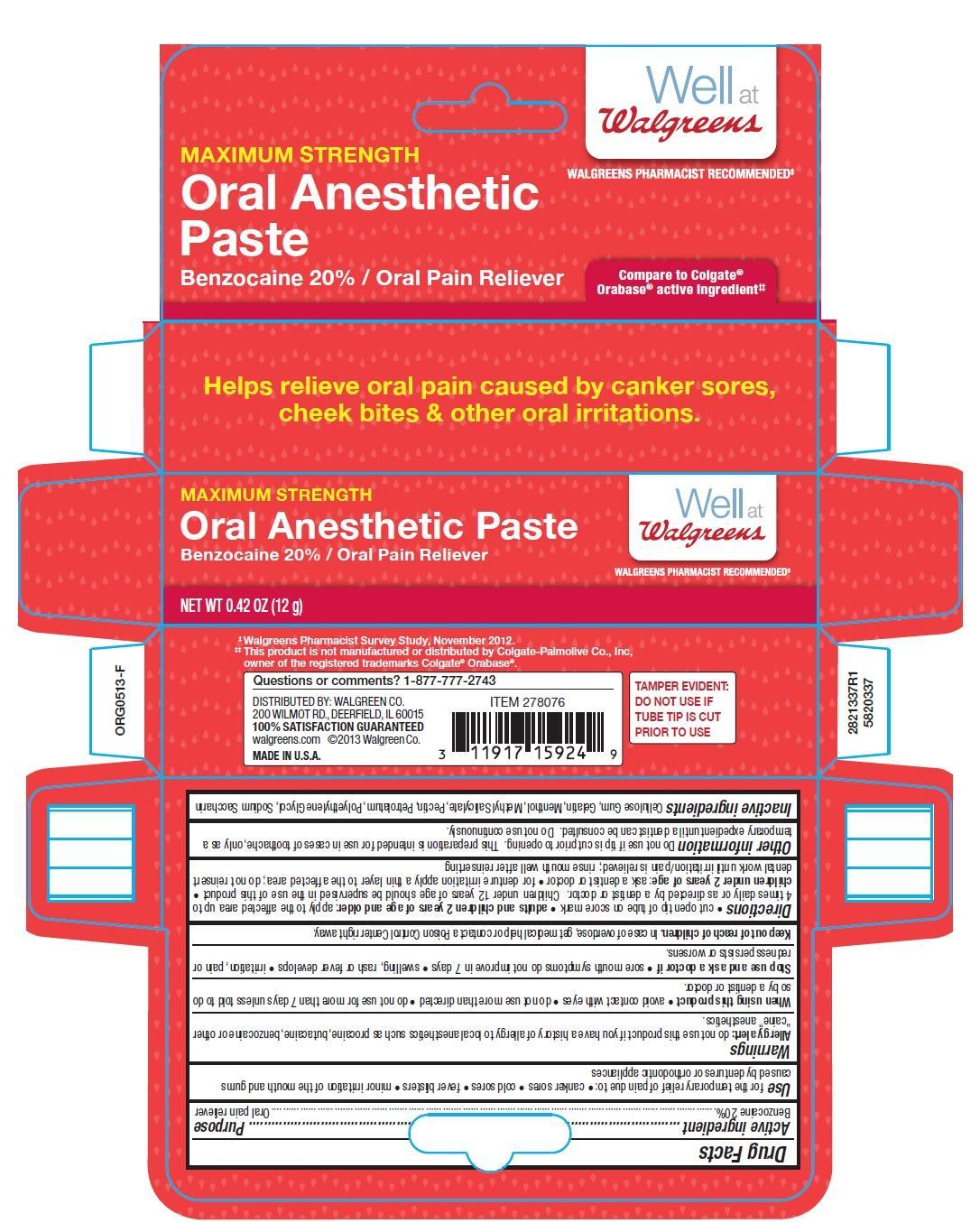
Use for the temporary relief of pain due to:
- canker sores
- cold sores
- fever blisters
- minor irritation of the mouth and gums
- minor irritation of the mouth and gums caused by dentures or orthodontic appliances
Warnings
Allergy alert: do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine or other “caine” anesthetics.
When using this product
- avoid contact with eyes
- do not use more than directed
- do not use for more than 7 days unless told to do so by a dentist or doctor.
Directions
- cut open tip of tube on score mark
- adults and children 2 years of age and older: apply to the affected area up to 4 times daily or as directed by a dentist or doctor. Children under 12 years of age should be supervised in the use of this product
- children under 2 years of age: ask a dentist or doctor
- for denture irritation apply a thin layer to the affected area; do not reinsert dental work until irritation/pain is relieved; rinse mouth well after reinserting
Other information Do not use if tip is cut prior to opening. This preparation is intended for use in cases of toothache, only as a
temporary expedient until a dentist can be consulted. Do not use continuously.
Inactive ingredients Cellulose Gum, Gelatin, Menthol, Methyl Salicylate, Pectin, Petrolatum, Polyethylene Glycol, Sodium Saccharin.