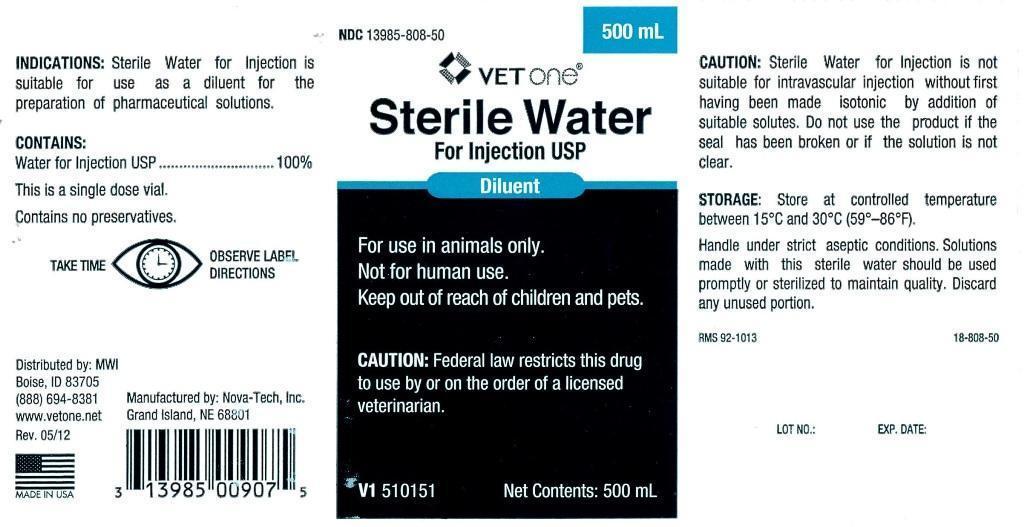
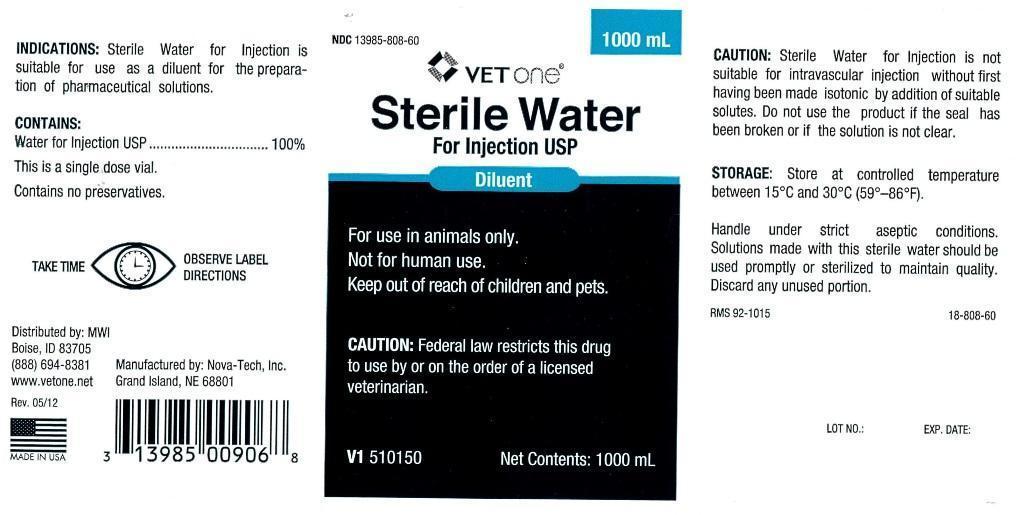
INDICATIONS:
Sterile Water for Injection is suitable for use as a diluent for the preparation of pharmaceutical solutions.
CONTAINS:
Water for Injection USP.............................100%
This is a single dose vial.
Contains no preservatives.
Distributed by: MWI
Boise, ID 83705
(888) 694-8381
www.vetone.net
Rev. 05/12
Manufactured by: Nova-Tech, Inc.
Grand Island, NE 68801
V1 501046
Net Contents: 250 mL
RMS 92-1011 18-808-25
LOT NO.: EXP. DATE:
CAUTION:
Sterile water for Injection is not suitable for intravascular injection without first having been made isotonic by addition of suitable solutes. Do not use the product if the seal has been broken or if the solution is not clear.