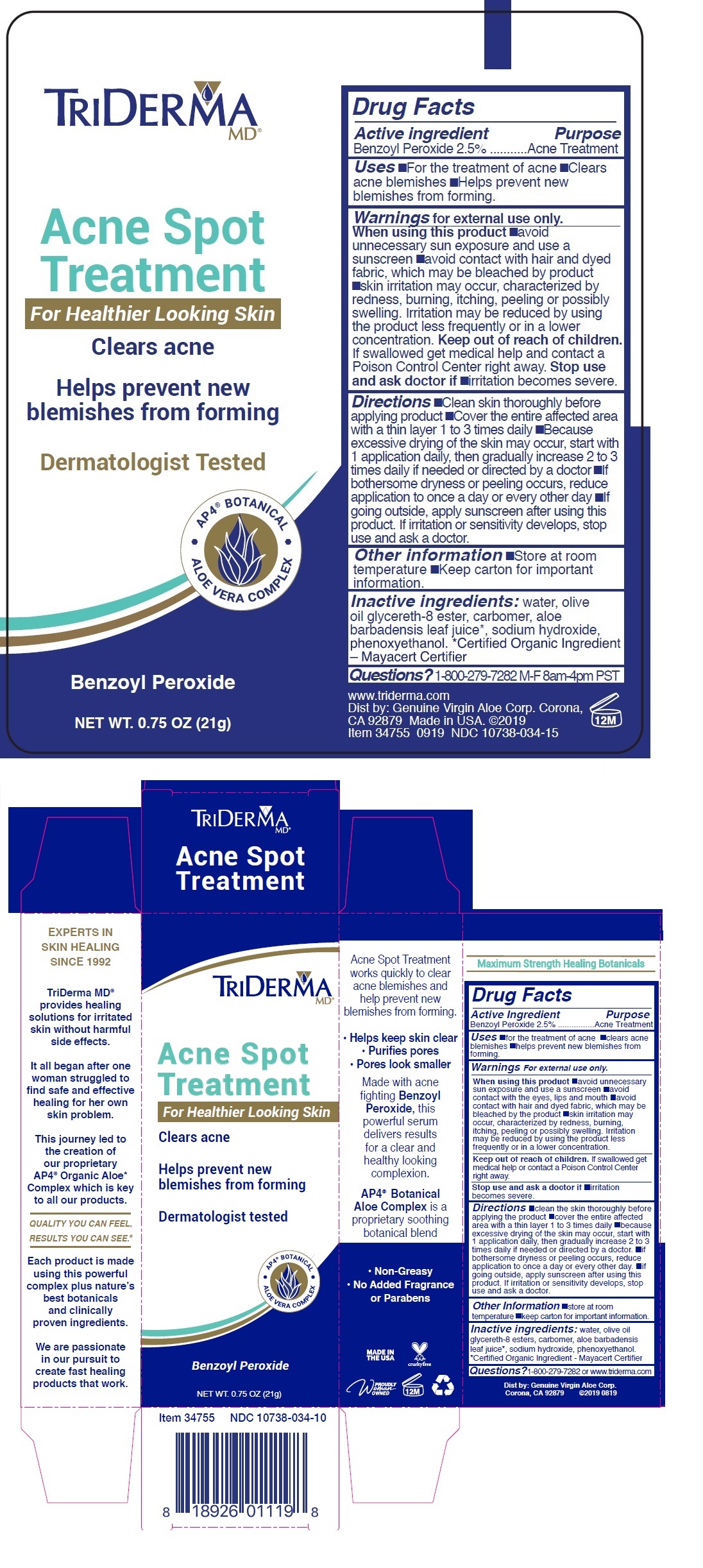
Warnings For external use only.
When using this product •avoid unnecessary sun exposure and use a sunscreen •avoid contact with the eyes, lips and mouth •avoid contact with hair and dyed fabric, which may be bleached by the product •skin irritation may occur, characterized by redness, burning, itching, peeling or possibly swelling. Irritation may be reduced by using the product less frequently or in a lower concentration.
Stop use and ask a doctor if •irritation becomes severe.
Directions •clean the skin thoroughly before applying the product •cover the entire affected area with a thin layer 1 to 3 times daily •because excessive drying of the skin may occur, start with 1 application daily, then gradually increase 2 to 3 times daily if needed or directed by a doctor •if bothersome dryness or peeling occurs, reduce application to once a day or every other day •if going outside, apply sunscreen after using this product. If irritation or sensitivity develops, stop use and ask a doctor.
Inactive ingredients: water, olive oil glycereth-8 esters, carbomer, aloe barbadensis leaf juice *, sodium hydroxide, phenoxyethanol. *Certified Organic Ingredient - Mayacert Certifier
For Healthier Looking Skin
Clears acne
Helps prevent new blemishes from forming
Dermatologist Tested
• AP4 ® BOTANICAL • ALOE VERA COMPLEX
Acne Spot Treatment works quickly to clear acne blemishes and help prevent new blemishes from forming.
• Helps keep skin clear
• Purifies pores
• Pores look smaller
Made with acne fighting Benzoyl Peroxide, this powerful serum delivers results for a clear and healthy looking complexion.
AP4 ® Botanical Aloe Complex is a proprietary soothing botanical blend
• Non-Greasy
• No Added Fragrance or Parabens
EXPERTS IN SKIN HEALING SINCE 1992
TriDerma MD ® provides healing solutions for irritated skin without harmful side effects.
It all began after one woman struggled to find safe and effective healing for her own skin problem.
This journey led to the creation of our proprietary AP4 ® Organic Aloe* complex which is key to all our products.
QUALITY YOU CAN FEEL, RESULTS YOU CAN SEE ®
Each product is made using this powerful complex plus nature's best botanicals and clinically proven ingredients.
We are passionate in our pursuit to create fast healing products that work.
MADE IN THE USA
Maximum Strength Healing Botanicals
Dist by: Genuine Virgin Aloe Corp. Corona,
CA 92879 ©2019 0819