INTERCEPTOR PLUS- milbemycin oxime and praziquantel tablet, chewable
Novartis Animal Health US, Inc.
----------
INTERCEPTOR®
PLUS
Description
INTERCEPTOR PLUS is available in four strengths in color-coded packages for oral administration to dogs and puppies according to their weight. Each chewable flavored tablet is formulated to provide a minimum of 0.23 mg/pound (0.5 mg/kg) of milbemycin oxime and 2.28 mg/pound (5 mg/kg) of praziquantel.
Milbemycin oxime consists of the oxime derivatives of 5-didehydromilbemycins in the ratio of approximately 80% A4 (C32H45NO7, MW 555.71) and 20% A3 (C31H43NO7, MW 541.68). Milbemycin oxime is classified as a macrocyclic anthelmintic.
Praziquantel is an isoquinolone anthelmintic with the chemical name 2-(Cyclohexylcarbonyl)-1,2,3,6,7,-11b-hexahydro-4H-pyrazino[2,1-a]isoquinolin-4-one.
Indications
INTERCEPTOR PLUS is indicated for the prevention of heartworm disease caused by Dirofilaria immitis; and for the treatment and control of adult roundworm (Toxocara canis, Toxascaris leonina), adult hookworm (Ancylostoma caninum), adult whipworm (Trichuris vulpis), and adult tapeworm (Taenia pisiformis, Echinococcus multilocularis and Echinococcus granulosus) infections in dogs and puppies two pounds of body weight or greater and six weeks of age and older.
Dosage and Administration
INTERCEPTOR PLUS should be administered orally, once every month, at the minimum dosage of 0.23 mg/lb (0.5 mg/kg) milbemycin oxime, and 2.28 mg/lb (5 mg/kg) praziquantel. For heartworm prevention, give once monthly for at least 6 months after exposure to mosquitoes (see EFFECTIVENESS).
| Body Weight | Milbemycin Oxime per chewable | Praziquantel per chewable | Number of chewables |
| 2 to 8 lbs. | 2.3 mg | 22.8 mg | One |
| 8.1 to 25 lbs. | 5.75 mg | 57 mg | One |
| 25.1 to 50 lbs. | 11.5 mg | 114 mg | One |
| 50.1 to 100 lbs. | 23 mg | 228 mg | One |
| Over 100 lbs. | Administer the appropriate combination of chewables. | ||
INTERCEPTOR PLUS may be offered to the dog by hand or added to a small amount of dog food. The chewables should be administered in a manner that encourages the dog to chew, rather than to swallow without chewing. Chewables may be broken into pieces and fed to dogs that normally swallow treats whole. Care should be taken that the dog consumes the complete dose, and treated animals should be observed for a few minutes after administration to ensure that no part of the dose is lost or rejected. If it is suspected that any of the dose has been lost, redosing is recommended.
Heartworm Prevention:
INTERCEPTOR PLUS should be administered at monthly intervals beginning within 1 month of the dog's first seasonal exposure to mosquitoes and continuing until at least 6 months after the dog's last seasonal exposure (see EFFECTIVENESS). INTERCEPTOR PLUS may be administered year-round without interruption. When switching from another heartworm preventative product to INTERCEPTOR PLUS, the first dose of INTERCEPTOR PLUS should be given within a month of the last dose of the former product.
Intestinal Nematode and Cestode Treatment and Control:
Dogs may be exposed to and can become infected with roundworms, whipworms, hookworms, and tapeworms throughout the year, regardless of season or climate. Clients should be advised of appropriate measures to prevent reinfection of their dog with intestinal parasites. Because the prepatent period for E. multilocularis may be as short as 26 days, dogs treated at the labeled monthly intervals may become reinfected and shed eggs between treatments.
Precautions
Treatment with fewer than 6 monthly doses after the last exposure to mosquitoes may not provide complete heartworm prevention (see EFFECTIVENESS).
Prior to administration of INTERCEPTOR PLUS, dogs should be tested for existing heartworm infections. At the discretion of the veterinarian, infected dogs should be treated to remove adult heartworms. INTERCEPTOR PLUS is not effective against adult D. immitis.
Mild, transient hypersensitivity reactions, such as labored breathing, vomiting, hypersalivation, and lethargy, have been noted in some dogs treated with milbemycin oxime carrying a high number of circulating microfilariae. These reactions are presumably caused by release of protein from dead or dying microfilariae.
Do not use in puppies less than six weeks of age.
Do not use in dogs or puppies less than two pounds of body weight.
The safety of INTERCEPTOR PLUS has not been evaluated in dogs used for breeding or in lactating females. Studies have been performed with milbemycin oxime alone (see ANIMAL SAFETY).
Adverse Reactions
The following adverse reactions have been reported in dogs after administration of milbemycin oxime or praziquantel: vomiting, diarrhea, depression/lethargy, ataxia, anorexia, convulsions, weakness, and salivation.
To report suspected adverse drug events, contact Novartis Animal Health at 800-637-0281 or the FDA at 1-888-FDA-VETS.
For technical assistance call Novartis Animal Health at 800-637-0281.
Information for Owner or Person Treating Animal:
Echinococcus multilocularis and Echinococcus granulosus are tapeworms found in wild canids and domestic dogs. E. multilocularis and E. granulosus can infect humans and cause serious disease (alveolar hydatid disease and hydatid disease, respectively).Owners of dogs living in areas where E. multilocularis or E. granulosus are endemic should be instructed on how to minimize their risk of exposure to these parasites, as well as their dog's risk of exposure. Although INTERCEPTOR PLUS was 100% effective in laboratory studies in dogs against E. multilocularis and E. granulosus, no studies have been conducted to show that the use of this product will decrease the incidence of alveolar hydatid disease or hydatid disease in humans. Because the prepatent period for E. multilocularis may be as short as 26 days, dogs treated at the labeled monthly intervals may become reinfected and shed eggs between treatments.
Effectiveness
Heartworm Prevention:
In a well-controlled laboratory study, INTERCEPTOR PLUS was 100% effective against induced heartworm infections when administered once monthly for 6 consecutive months. In well-controlled laboratory studies, neither one dose nor two consecutive doses of INTERCEPTOR PLUS provided 100% effectiveness against induced heartworm infections.
Intestinal Nematodes and Cestodes Treatment and Control:
Elimination of the adult stage of hookworm (Ancylostoma caninum), roundworm (Toxocara canis, Toxascaris leonina), whipworm (Trichuris vulpis) and tapeworm (Echinococcus multilocularis, Echinococcus granulosus, Taenia pisiformis) infections in dogs was demonstrated in well-controlled laboratory studies.
Animal Safety
INTERCEPTOR PLUS:
In a repeated dose safety study, 40 ten-week-old puppies (10 per group) were dosed with either a sham dose (0X) or 1, 3, or 5X the maximum label exposure of INTERCEPTOR PLUS every 14 days for a total of seven treatments. Ataxia, lethargy, and salivation were seen in the 3X and 5X treated dogs following each of the seven doses. Vomiting was seen in all treatment groups but had a higher incidence in the 3X and 5X treatment groups.
In a repeated dose safety study, 64 six-week-old puppies (16 per group) were dosed with either a sham dose (0X) or 1, 3, or 5X the maximum label exposure of INTERECEPTOR PLUS every 14 days for a total of four treatments. Lethargy was observed in all groups. Ataxia was observed in the three treated groups, including one dog in the 1X treated group. For both lethargy and ataxia the incidence and duration increased in the 3X and 5X groups. These signs were observed during the first 24 hours following treatment. Salivation and tremors were observed in the 3X and 5X treated dogs beginning immediately after dosing and up to six hours post dose. Vomiting was only observed in the 5X treatment group on most, but not all, treatment days.
For INTERCEPTOR PLUS the maximum exposure based on product dosing is 2.5 mg/kg for milbemycin oxime and 25.1 mg/kg for praziquantel, which is higher than the minimum effective dose used in the safety studies for milbemycin oxime (see below).
Milbemycin Oxime:
Two studies were conducted in heartworm-infected dogs treated with milbemycin oxime. Mild, transient hypersensitivity reactions were observed in dogs with high microfilariae counts (see PRECAUTIONS).
Safety studies in pregnant dogs demonstrated that doses of 0.6X the maximum exposure dose of INTERCEPTOR PLUS, (1.5 mg/kg of milbemycin oxime), administered daily from mating through weaning, resulted in measurable concentrations of milbemycin oxime in milk. Puppies nursing these females demonstrated milbemycin oxime-related effects (depression, decreased activity, diarrhea, dehydration, nasal discharge). A subsequent study, which evaluated the daily administration of 0.6X the maximum exposure dose of INTERCEPTOR PLUS, from mating until one week before weaning, demonstrated no effects on the pregnant females or their litters. A study, in which pregnant females were dosed once, at 0.6X the maximum exposure dose of INTERCEPTOR PLUS before, on the day of, or shortly after whelping, resulted in no effects on the puppies.
Some nursing puppies, at 2, 4, and 6 weeks of age, administered oral doses of 9.6 mg/kg milbemycin oxime (3.8X the maximum exposure dose of INTERCEPTOR PLUS) exhibited tremors, vocalization, and ataxia. These effects were all transient and puppies returned to normal within 24 to 48 hours. No effects were observed in puppies administered 0.5 mg/kg milbemycin oxime (minimum label dose).
A rising-dose safety study conducted in rough-coated Collies resulted in ataxia, pyrexia, and periodic recumbency in one of fourteen dogs administered milbemycin oxime at 12.5 mg/kg (5X the maximum exposure dose of INTERCEPTOR PLUS). Prior to receiving the 12.5 mg/kg dose on day 56 of the study, all animals had undergone a dosing regimen consisting of 2.5 mg/kg milbemycin oxime on day 0, followed by 5.0 mg/kg on day 14, and 10.0 mg/kg on day 32. No adverse reactions were observed in any of the Collies treated with doses less than 12.5 mg/kg.
How Supplied
INTERCEPTOR PLUS is available in four strengths, formulated according to the weight of the dog. Each strength is available in color-coded packages of one, six, or twelve chewable tablets each.
Manufactured for: Novartis Animal Health US, Inc.
Greensboro, NC 27408, USA
NADA #141-338, Approved by FDA
© 2014 Novartis Animal Health US, Inc.
NAH/INT-PLUS/PI/3
01/15
46154412
NOVARTIS
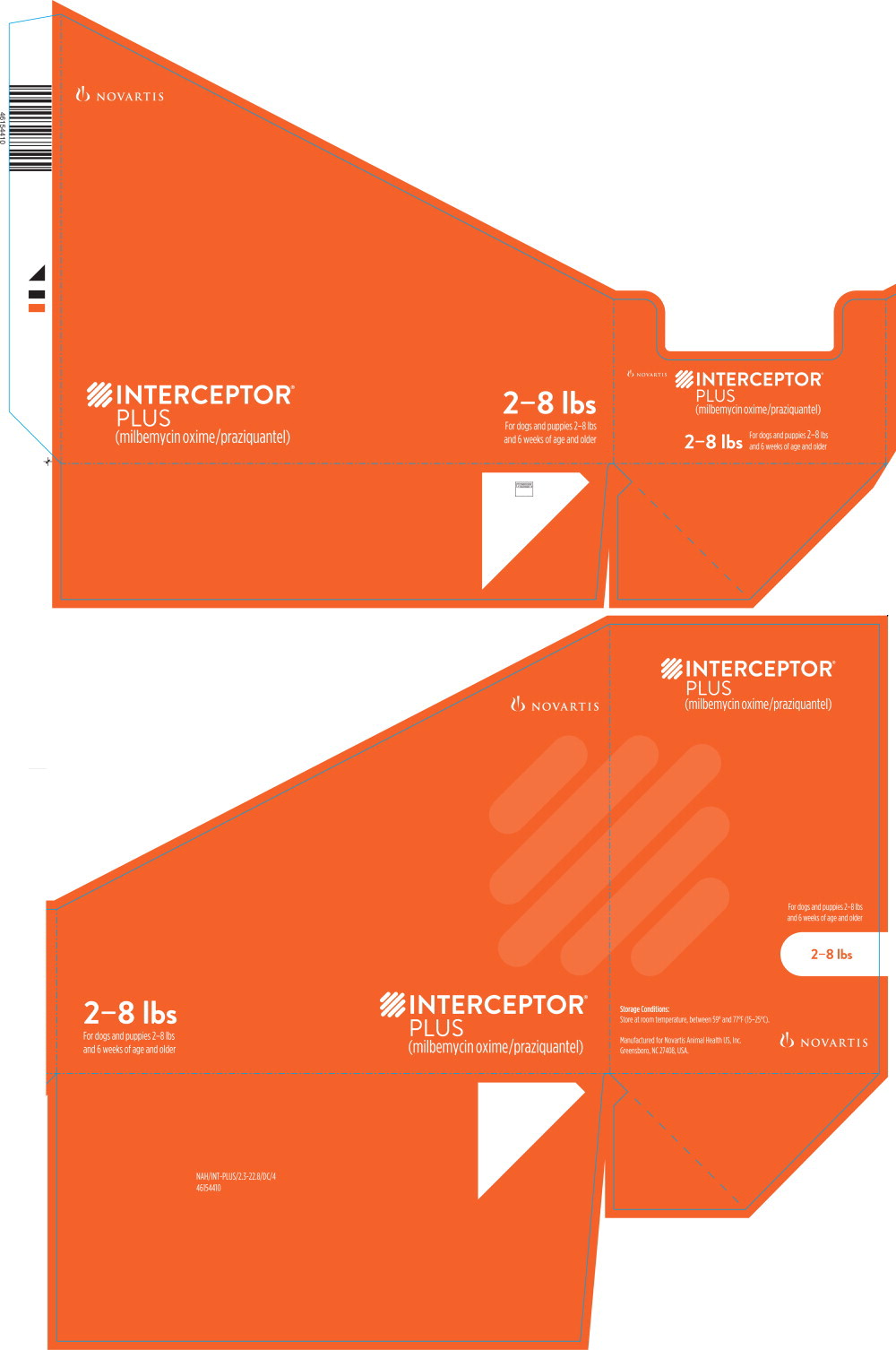
Principal Display Panel - 2-8 lbs Tray Label
NOVARTIS
INTERCEPTOR®
PLUS
(milbemycin oxime/praziquantel)
2-8 lbs
For dogs and puppies 2-8 lbs
and 6 weeks of age and older
Principal Display Panel - 2-8 lbs Carton Label
INTERCEPTOR®
PLUS
(milbemycin oxime/praziquantel)
6
CHEWABLES
Each containing 2.3 mg
milbemycin oxime
and 22.8 mg praziquantel
For dogs and puppies 2-8 lbs
and 6 weeks of age and older
2-8 lbs
Caution: Federal (USA) law restricts this drug to use by or on the order of a
licensed veterinarian. Keep this and all drugs out of reach of children.
BROAD-SPECTRUM PARASITICIDE
- Prevents heartworm disease
- Treats and controls common intestinal parasites,
including roundworms, hookworms, whipworms
and tapeworms
NOVARTIS
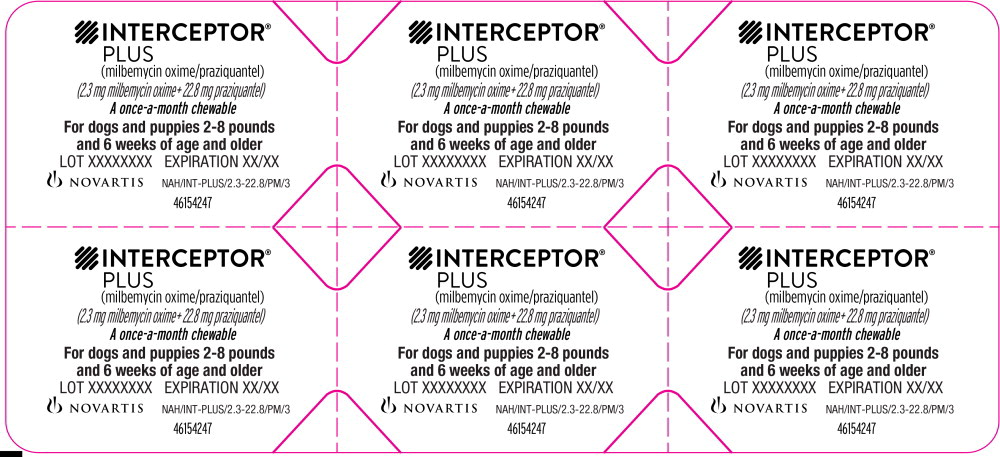
Principal Display Panel - 2-8 lbs Blister Pack Label
INTERCEPTOR®
PLUS
(milbemycin oxime/praziquantel)
(2.3 mg milbemycin oxime + 22.8 mg praziquantel)
A once-a-month chewable
For dogs and puppies 2-8 pounds
and 6 weeks of age and older
LOT XXXXXXXX EXPIRATION XX/XX
NOVARTIS NAH/INT-PLUS/2.3-22.8/PM/3
46154247
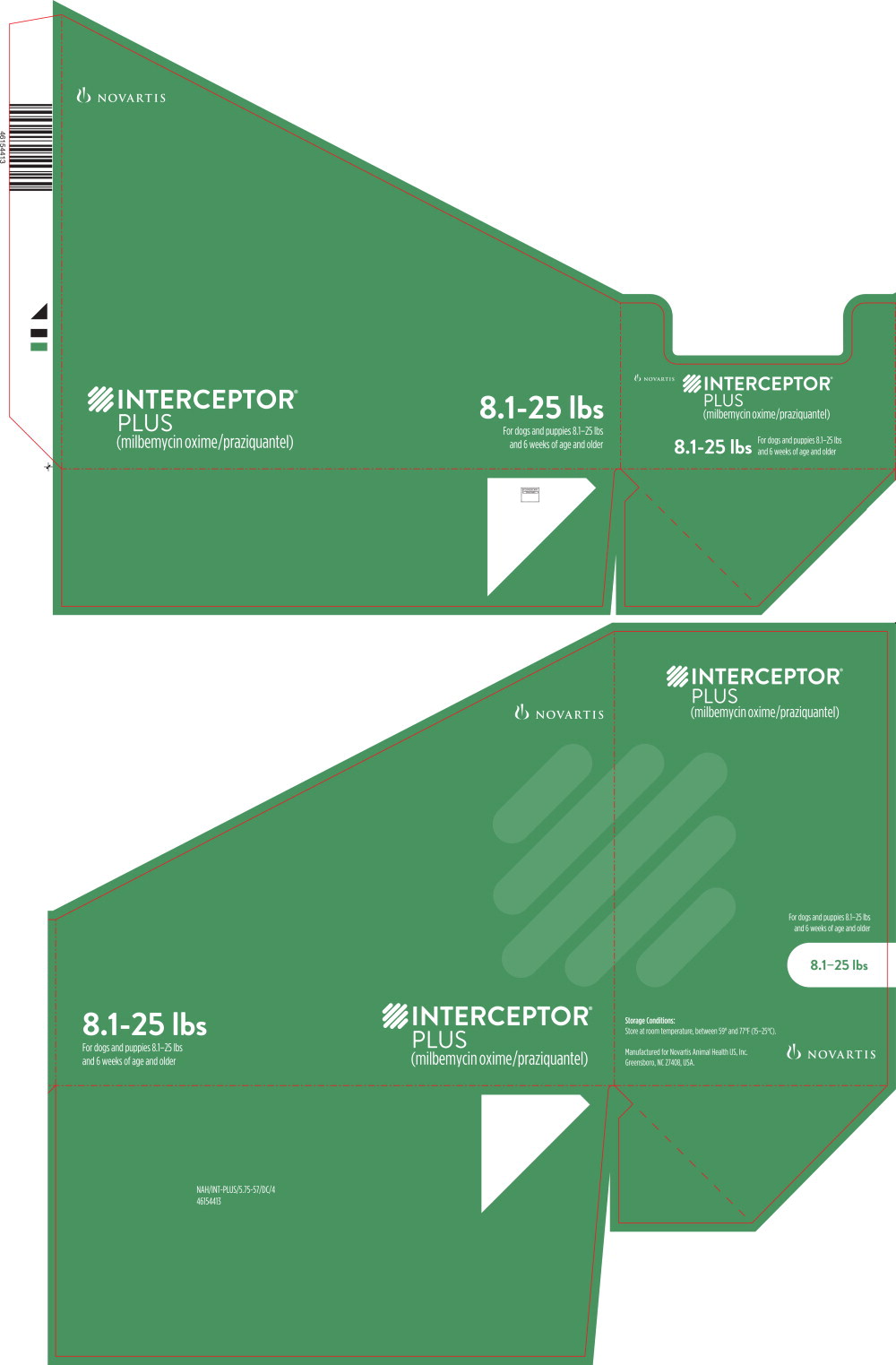
Principal Display Panel - 8.1-25 lbs Tray Label
NOVARTIS
INTERCEPTOR®
PLUS
(milbemycin oxime/praziquantel)
8.1-25 lbs
For dogs and puppies 8.1-25 lbs
and 6 weeks of age and older
Principal Display Panel - 8.1-25 lbs Carton Label
INTERCEPTOR®
PLUS
(milbemycin oxime/praziquantel)
6
CHEWABLES
Each containing 5.75 mg
milbemycin oxime
and 57 mg praziquantel
For dogs and puppies 8.1-25 lbs
and 6 weeks of age and older
8.1-25 lbs
Caution: Federal (USA) law restricts this drug to use by or on the order of a
licensed veterinarian. Keep this and all drugs out of reach of children.
BROAD-SPECTRUM PARASITICIDE
- Prevents heartworm disease
- Treats and controls common intestinal parasites,
including roundworms, hookworms, whipworms
and tapeworms
NOVARTIS
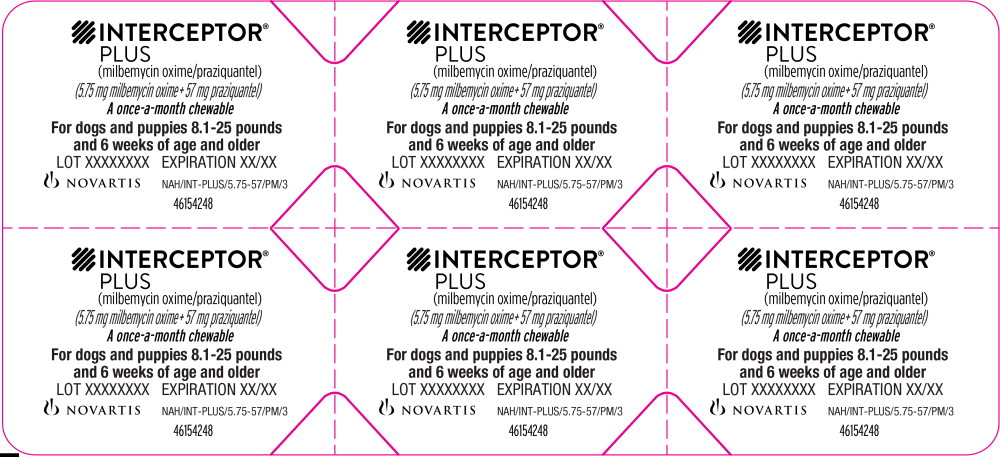
Principal Display Panel - 8.1-25 lbs Blister Pack Label
INTERCEPTOR®
PLUS
(milbemycin oxime/praziquantel)
(5.75 mg milbemycin oxime + 57 mg praziquantel)
A once-a-month chewable
For dogs and puppies 8.1-25 pounds
and 6 weeks of age and older
LOT XXXXXXXX EXPIRATION XX/XX
NOVARTIS NAH/INT-PLUS/5.75-57/PM/3
46154248
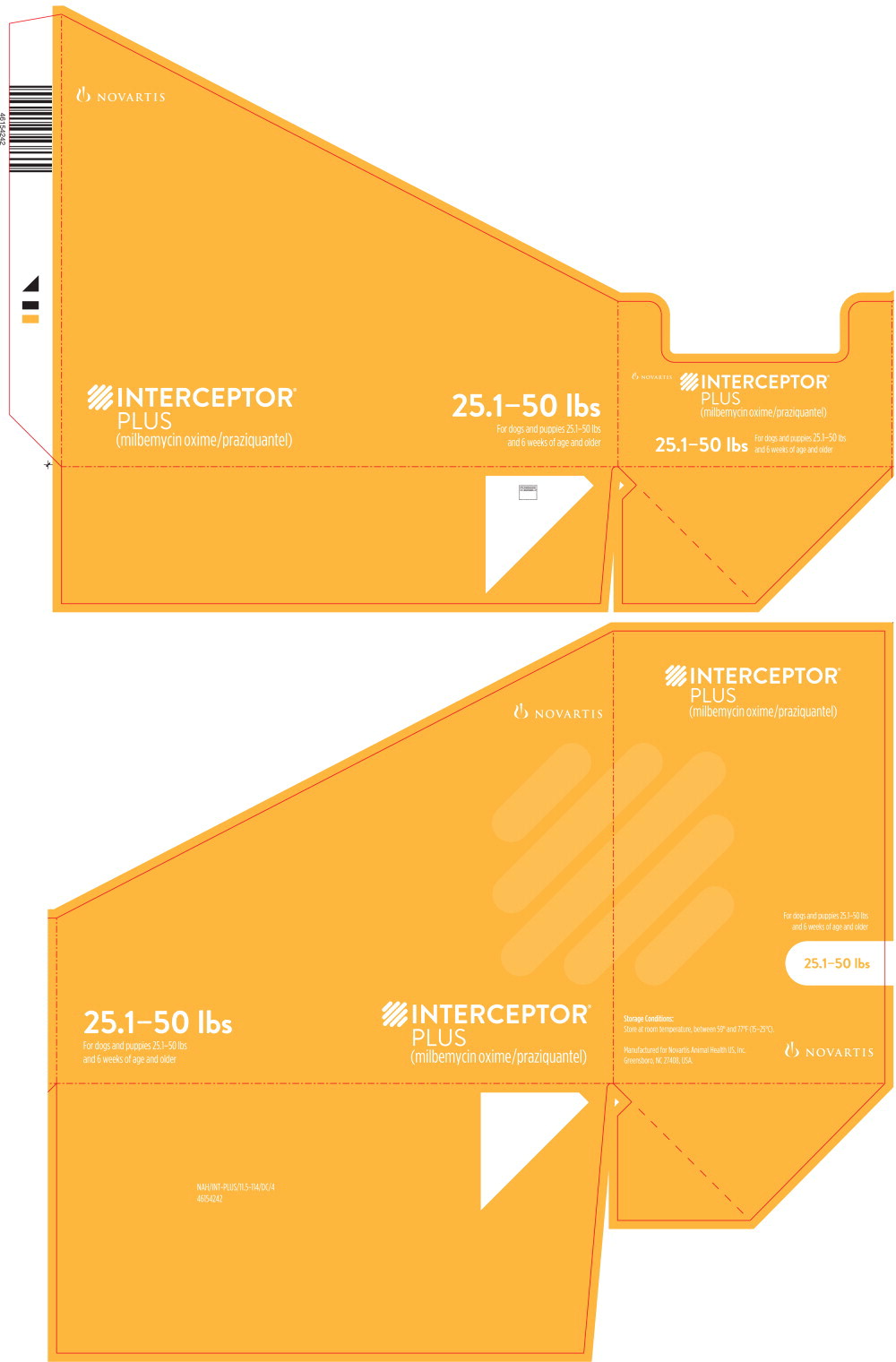
Principal Display Panel - 25.1-50 lbs Tray Label
NOVARTIS
INTERCEPTOR®
PLUS
(milbemycin oxime/praziquantel)
25.1-50 lbs
For dogs and puppies 25.1-50 lbs
and 6 weeks of age and older
Principal Display Panel - 25.1-50 lbs Carton Label
INTERCEPTOR®
PLUS
(milbemycin oxime/praziquantel)
6
CHEWABLES
Each containing 11.5 mg
milbemycin oxime
and 114 mg praziquantel
For dogs and puppies 25.1-50 lbs
and 6 weeks of age and older
25.1-50 lbs
Caution: Federal (USA) law restricts this drug to use by or on the order of a
licensed veterinarian. Keep this and all drugs out of reach of children.
BROAD-SPECTRUM PARASITICIDE
- Prevents heartworm disease
- Treats and controls common intestinal parasites,
including roundworms, hookworms, whipworms
and tapeworms
NOVARTIS
Principal Display Panel - 25.1-50 lbs Blister Pack Label
INTERCEPTOR®
PLUS
(milbemycin oxime/praziquantel)
(11.5 mg milbemycin oxime + 114 mg praziquantel)
A once-a-month chewable
For dogs and puppies 25.1-50 pounds
and 6 weeks of age and older
LOT XXXXXXXX EXPIRATION XX/XX
NOVARTIS NAH/INT-PLUS/11.5-114/PM/3
46154249
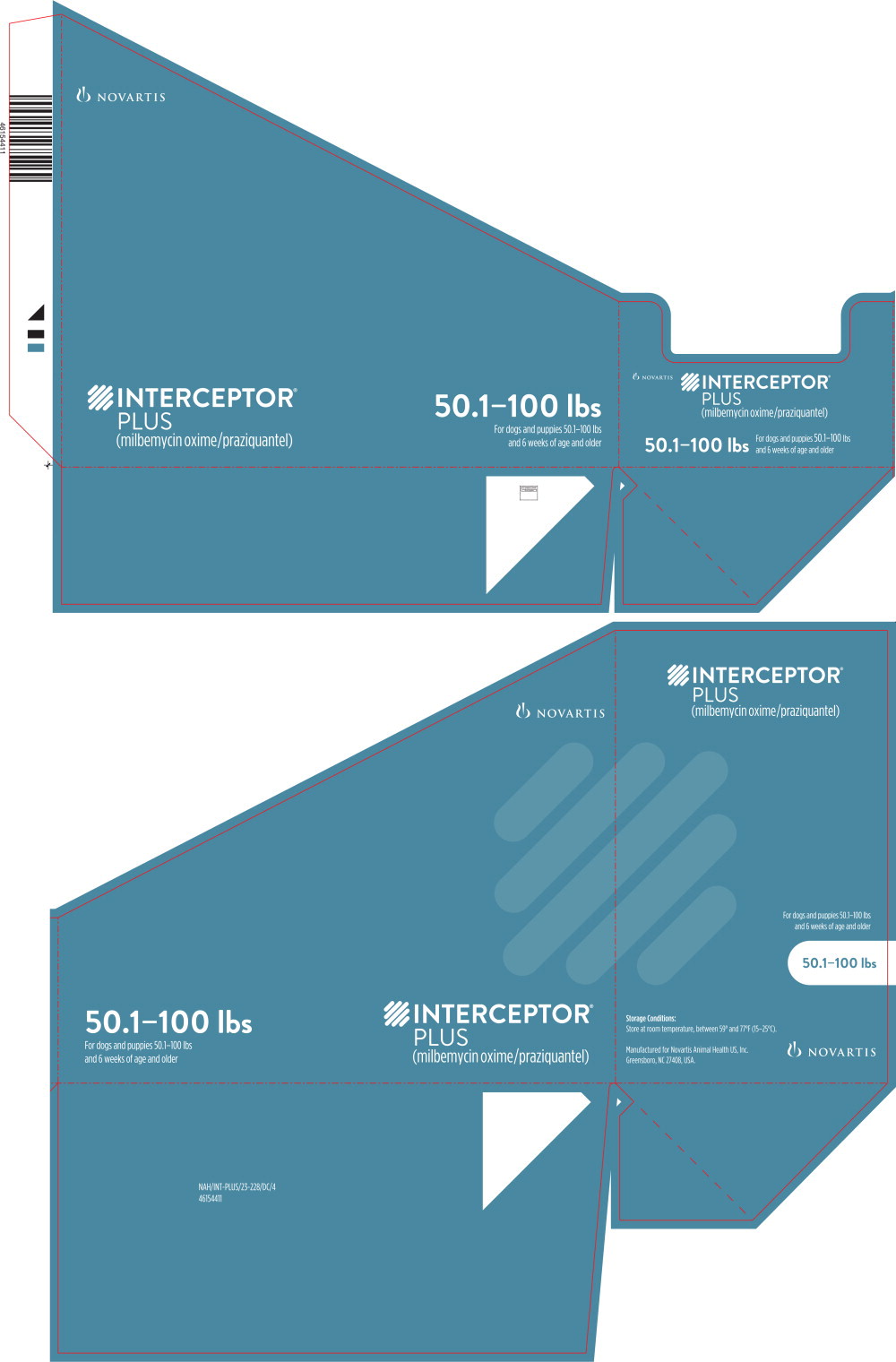
Principal Display Panel - 50.1-100 lbs Tray Label
NOVARTIS
INTERCEPTOR®
PLUS
(milbemycin oxime/praziquantel)
50.1-100 lbs
For dogs and puppies 50.1-100 lbs
and 6 weeks of age and older
Principal Display Panel - 50.1-100 lbs Carton Label
INTERCEPTOR®
PLUS
(milbemycin oxime/praziquantel)
6
CHEWABLES
Each containing 23 mg
milbemycin oxime
and 228 mg praziquantel
For dogs and puppies 50.1-100 lbs
and 6 weeks of age and older
50.1-100 lbs
Caution: Federal (USA) law restricts this drug to use by or on the order of a
licensed veterinarian. Keep this and all drugs out of reach of children.
BROAD-SPECTRUM PARASITICIDE
- Prevents heartworm disease
- Treats and controls common intestinal parasites,
including roundworms, hookworms, whipworms
and tapeworms
NOVARTIS
Principal Display Panel - 50.1-100 lbs Blister Pack Label
INTERCEPTOR®
PLUS
(milbemycin oxime/praziquantel)
(23 mg milbemycin oxime + 228 mg praziquantel)
A once-a-month chewable
For dogs and puppies 50.1-100 pounds
and 6 weeks of age and older
LOT XXXXXXXX EXPIRATION XX/XX
NOVARTIS NAH/INT-PLUS/23.0-228/PM/3
46154400
| INTERCEPTOR PLUS
milbemycin oxime and praziquantel tablet, chewable |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| INTERCEPTOR PLUS
milbemycin oxime and praziquantel tablet, chewable |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| INTERCEPTOR PLUS
milbemycin oxime and praziquantel tablet, chewable |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| INTERCEPTOR PLUS
milbemycin oxime and praziquantel tablet, chewable |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Novartis Animal Health US, Inc. (966985624) |