SKIN SO SOFT ULTRA EVEN ROLL-ON ANTI-PERSPIRANT DEODORANT- aluminum chlorohydrate gel
New Avon LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
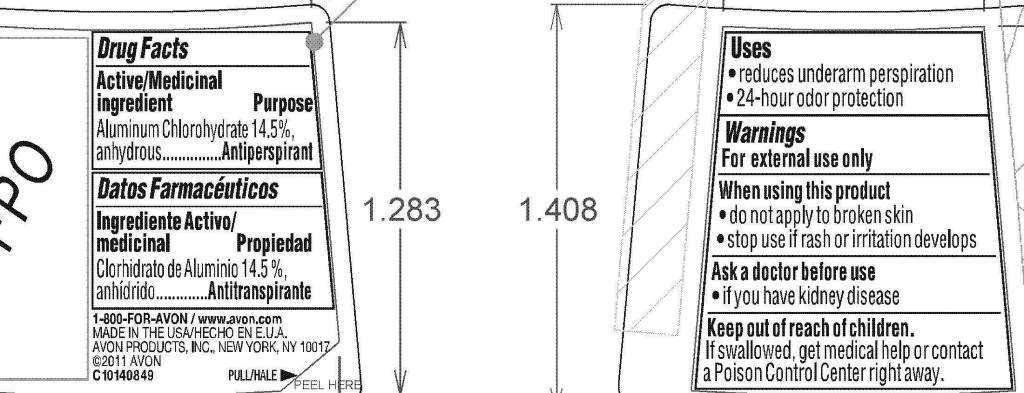
Active/Medicinal ingredient
Aluminum Chlorohydrate 14.5%, anhydrous............
Purpose.............Antiperspirant
Uses
• reduces underarm perspiration
• 24-hour odor protection
Warnings
For external use only
When using this product
• do not apply to broken skin
• stop use if rash or irritation develops
Ask a doctor before use
• if you have kidney disease
Keep out of reach of children.
If swallowed, get medical help or
contact a Poison Control Center
right away.
Directions
• Apply to underarms only.
INACTIVE/NON-MEDICINAL INGREDIENTS:
WATER/EAU
STEARETH-2
PPG-15 STEARYL ETHER
DICAPRYL ADIPATE
HYDROLYZED SILK
HYDROLYZED KERATIN
SAXIFRAGA SARMENTOSA EXTRACT
YEAST EXTRACT/EXTRAIT DE LEVURE
VITIS VINIFERA (GRAPE) FRUIT EXTRACT
MORUS NIGRA ROOT EXTRACT
SCUTELLARIA BAICALENSIS ROOT EXTRACT
TOCOPHERYL ACETATE
PPG-12/SMDI COPOLYMER
STEARETH-20
ISOPROPYL PALMITATE
PARFUM/FRAGRANCE
Questions? Call 1-800-FOR-AVON