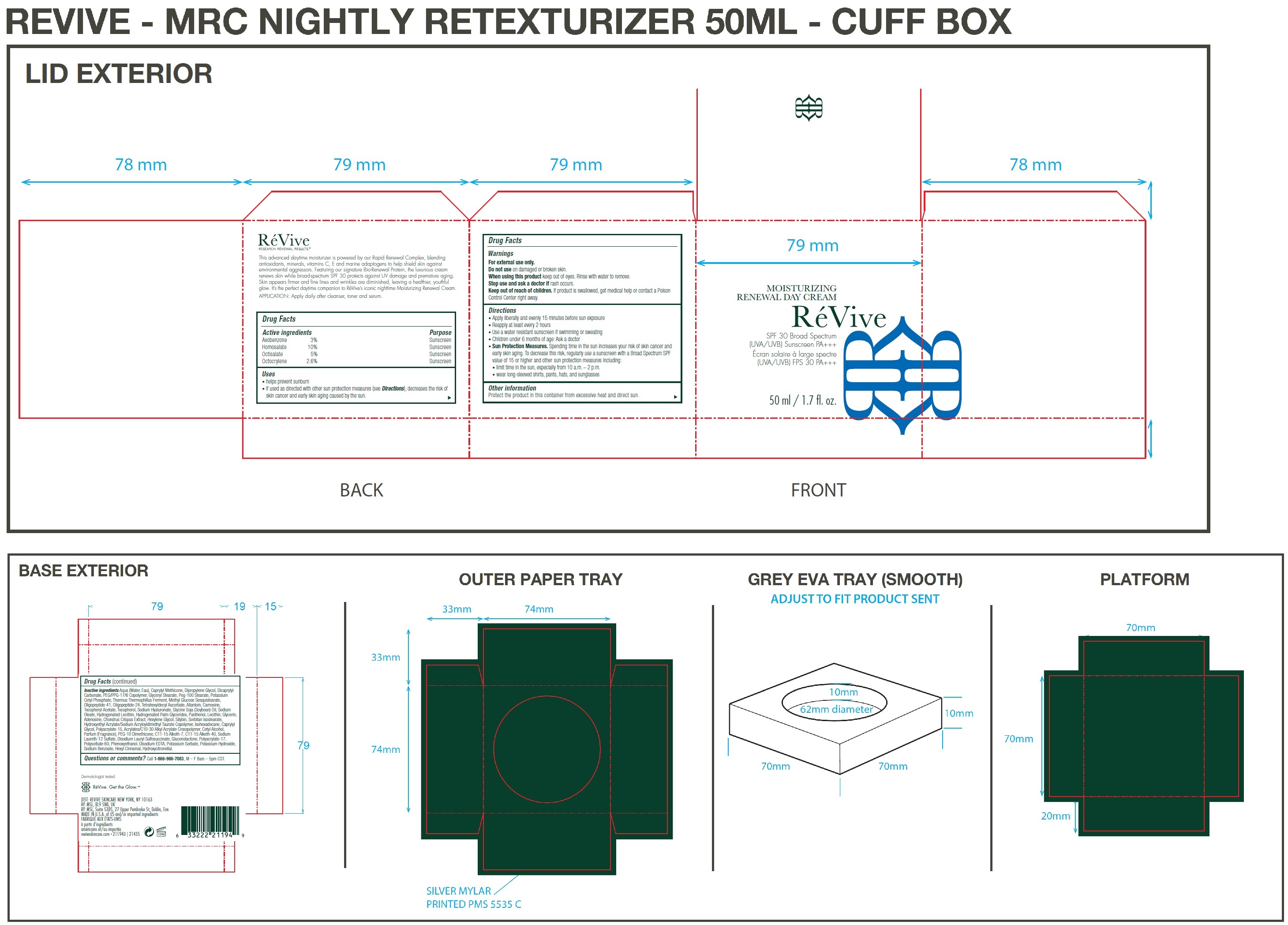
Uses
• helps prevent sunburn
• if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
Directions
• Apply liberally and evenly 15 minutes before sun exposure • Reapply at least every 2 hours • Use a water resistant sunscreen if swimming or sweating • Children under 6 months of age: Ask a doctor • Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including: • limit time in the sun, especially from 10 a.m. – 2 p.m. • wear long-sleeved shirts, pants, hats, and sunglasses
Sun Protection Measures.
Inactive ingredients:
Aqua (Water, Eau), Caprylyl Methicone, Dipropylene Glycol, Dicaprylyl Carbonate, PEG/PPG-17/6 Copolymer, Glyceryl Stearate, Peg-100 Stearate, Potassium Cetyl Phosphate, Thermus Thermophillus Ferment, Methyl Glucose Sesquistearate, Oligopeptide-41, Oligopeptide-24, Tetrahexyldecyl Ascorbate, Allantoin, Carnosine, Tocopheryl Acetate, Tocopherol, Sodium Hyaluronate, Glycine Soja (Soybean) Oil, Sodium Oleate, Hydrogenated Lecithin, Hydrogenated Palm Glycerides, Panthenol, Lecithin, Glycerin, Adenosine, Chondrus Crispus Extract, Hexylene Glycol, Silybin, Sorbitan Isostearate, Hydroxyethyl Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Isohexadecane, Caprylyl Glycol, Polyacrylate-15, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Cetyl Alcohol, Parfum (Fragrance), PEG-10 Dimethicone, C11-15 Alketh-7, C11-15 Alketh-40, Sodium Laureth-12 Sulfate, Disodium Lauryl Sulfosuccinate, Gluconolactone, Polyacrylate-17, Polysorbate 60, Phenoxyethanol, Disodium EDTA, Potassium Sorbate, Potassium Hydroxide, Sodium Benzoate, Hexyl Cinnamal, Hydroxycitronellal.