PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 65841-606-16 in bottle of 90 Capsules
Atomoxetine Capsules, 18 mg
90 Capsules

NDC 65841-607-16 in bottle of 90 Capsules
Atomoxetine Capsules, 25 mg
90 Capsules

NDC 65841-608-16 in bottle of 90 Capsules
Atomoxetine Capsules, 40 mg
90 Capsules

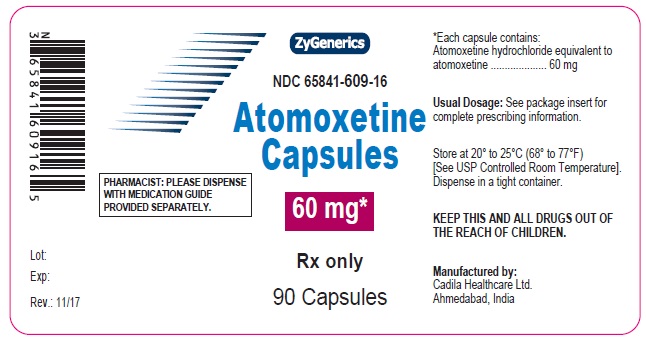
NDC 65841-609-16 in bottle of 90 Capsules
Atomoxetine Capsules, 60 mg
90 Capsules
NDC 65841-610-16 in bottle of 90 Capsules
Atomoxetine Capsules, 80 mg
90 Capsules

NDC 65841-611-16 in bottle of 90 Capsules
Atomoxetine Capsules, 100 mg
90 Capsules

NDC 65841-605-16 in bottle of 90 Capsules
Atomoxetine Capsules, 10 mg
90 Capsules
