PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
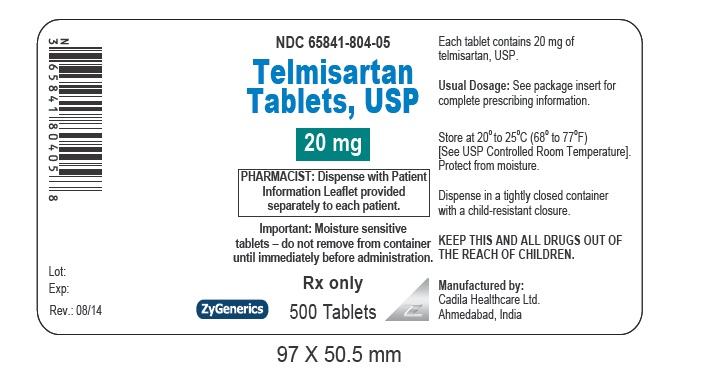
NDC 65841-804-05 in bottle of 500 tablets
Telmisartan Tablets USP, 20 mg
Rx only
500 tablets
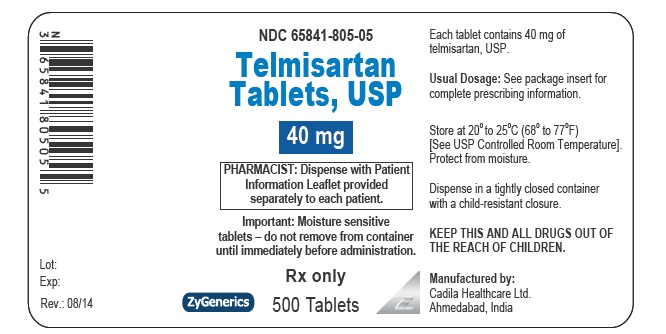
NDC 65841-805-05 in bottle of 500 tablets
Telmisartan Tablets USP, 40 mg
Rx only
500 tablets
NDC 65841-806-05 in bottle of 500 tablets
Telmisartan Tablets USP, 80 mg
Rx only
500 tablets
