Uses
- for the temporary relief of burning and irritation due to dryness of the eye
- for the temporary relief of discomfort due to minor irritations of the eye or to exposure to wind or sun
- for use as a protectant against further irritation or to relieve dryness of the eye
- for use as a lubricant to prevent further irritation or to relieve dryness of the eye
Warnings
For external use only
When using this product
- do not touch tip of container to any surface to avoid contamination
- replace cap after each use
Inactive Ingredients
boric acid, dimyristoyl phosphatidylglycerol, edetate disodium, hydroxypropyl guar, mineral oil, polyoxyl 40 stearate, POLYQUAD® (polyquaternium-1) 0.001% preservative, sorbitan tristearate, sorbitol and purified water. May contain hydrochloric acid and/or sodium hydroxide to adjust pH.
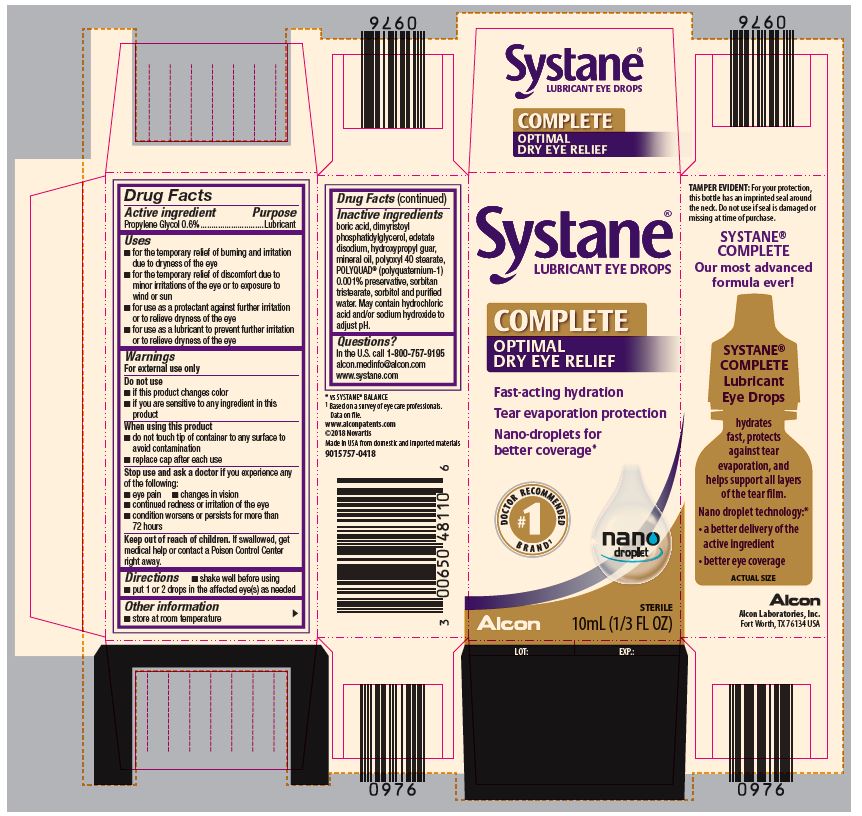
PRINCIPAL DISPLAY PANEL
Systane®
LUBRICANT EYE DROPS
COMPLETE
OPTIMAL DRY EYE RELIEF
Fast-acting hydration
Tear evaporation protection
Nano-droplets for better coverage*
#1 DOCTOR RECOMMENDED BRAND1
nano droplet
Alcon
STERILE
10mL (1/3 FL OZ)
LOT: EXP.:
SIDE PANEL
TAMPER EVIDENT: For your protection,
this bottle has an imprinted seal around
the neck. Do not use if seal is damaged or
missing at time of purchase.
SYSTANE®
COMPLETE
Our most advanced
formula ever!
SYSTANE®
COMPLETE
Lubricant
Eye Drops
hydrates
fast, protects
against tear
evaporation, and
helps support all layers
of the tear film.
Nano droplet technology:*
● a better delivery of the
active ingredient
● better eye coverage
ACTUAL SIZE
Alcon
Alcon Laboratories, Inc.
Fort Worth, TX 76143 USA
* vs SYSTANE® BALANCE
1Based on a survey of eye care professionals.
Data on file.
www.alconpatents.com
Made in USA from domestic and imported materials
300048645-0621

Systane®
LUBRICANT EYE DROPS
COMPLETE
OPTIMAL DRY EYE RELIEF
Fast-acting hydration
Tear evaporation protection
Nano-droplets for better coverage*
#1 DOCTOR RECOMMENDED BRAND1
nano droplet
Alcon
STERILE
10mL (1/3 FL OZ)
SIDE PANEL
TAMPER EVIDENT: For your protection, this bottle has an imprinted seal around the neck. Do not use if seal is damaged or missing at time of purchase.
Systane®COMPLETE
Our most advanced formula ever!
Systane® COMPLETE Lubricant Eye Drops hydrates fast, protects against tear evaporation, and helps support all layers of the tear film.
Nano droplet technology*
● a better delivery of the active ingredient
● better eye coverage
* vs Systane®BALANCE
1 Based on a survey of eye care professionals. Data on file.
www.alconpatents.com
©2018 Novartis
Made in USA from domestic and imported materials
9015757-0418
Alcon
Alcon Laboratories, Inc.
Fort Worth, TX 76143 USA