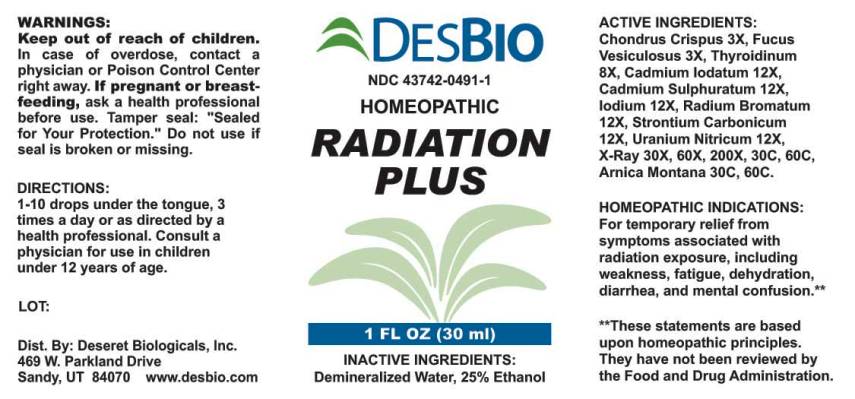
RADIATION PLUS- chondrus crispus, fucus vesiculosus, thyroidinum (suis), cadmium iodatum, cadmium sulphuratum, iodium, uranium nitricum, radium bromatum, strontium carbonicum, x-ray, arnica montana liquid
Deseret Biologicals, Inc.
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Drug Facts:
ACTIVE INGREDIENTS:
Chondrus crispus 3X, Fucus vesiculosus 3X, Thyroidinum (Suis) 8X, Cadmium iodatum 12X, Cadmium sulphuratum 12X, Iodium 12X, Uranium nitricum 12X, Radium bromatum 12X, Strontium carbonicum 12X, X-Ray 30X, 60X, 200X, 30C, 60C, Arnica Montana 30C, 60C.
HOMEOPATHIC INDICATIONS:
For temporary relief from symptoms associated with radiation exposure, including weakness, fatigue, dehydration, diarrhea, and mental confusion.**
**These statements are based upon traditional homeopathic principles. They have not been reviewed by the Food and Drug Administration.
WARNINGS:
Keep out of reach of children. In case of overdose, contact physician or Poison Control Center right away.
If pregnant or breast-feeding, ask a health professional before use.
Tamper seal: "Sealed for Your Protection." Do not use if seal is broken or missing.
KEEP OUT OF REACH OF CHILDREN.
KEEP OUT OF REACH OF CHILDREN. In case of overdose, contact physician or Poison Control Center right away.
DIRECTIONS:
1-10 drops under the tongue, 3 times a day or as directed by a health professional. Consult a physician for use in children under 12 years of age.
HOMEOPATHIC INDICATIONS:
For temporary relief from symptoms associated with radiation exposure, including weakness, fatigue, dehydration, diarrhea, and mental confusion.**
**These statements are based upon traditional homeopathic principles. They have not been reviewed by the Food and Drug Administration.
| RADIATION PLUS
chondrus crispus, fucus vesiculosus, thyroidinum (suis), cadmium iodatum, cadmium sulphuratum, iodium, uranium nitricum, radium bromatum, strontium carbonicum, x-ray, arnica montana liquid |
|||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||
| Labeler - Deseret Biologicals, Inc. (940741853) |
| Registrant - Apotheca Company (844330915) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Apotheca Company | 844330915 | manufacture(43742-0491) , api manufacture(43742-0491) , label(43742-0491) , pack(43742-0491) | |