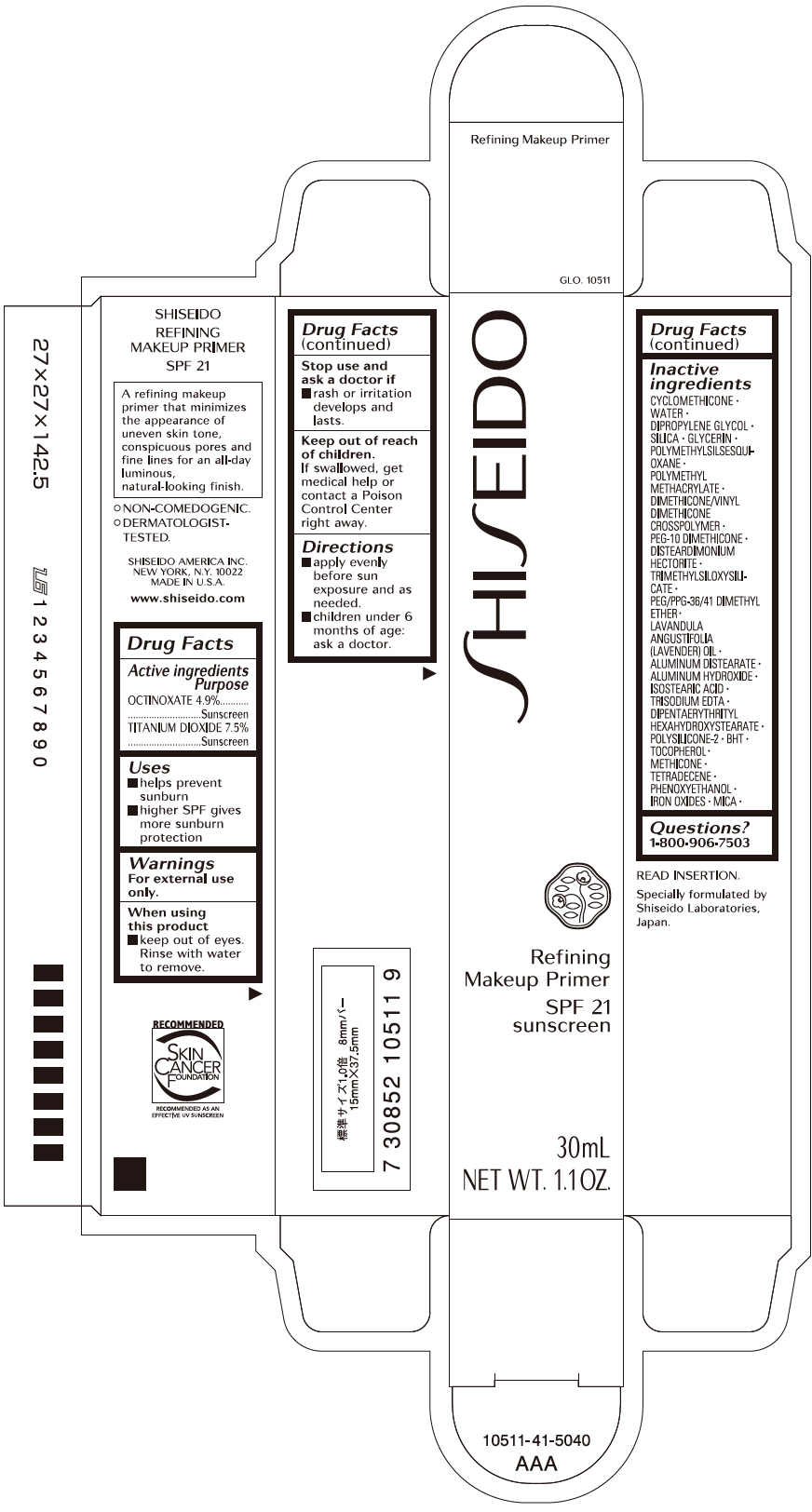
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
Directions
For sunscreen use:
- apply every before sun exposure and as needed
- children under 6 months: Ask a doctor
- reapply at least every two hour
Inactive Ingredients
CYCLOMETHICONE • WATER • DIPROPYLENE GLYCOL • SILICA • GLYCERIN • POLYMETHYLSILSESQUIOXANE • POLYMETHYL METHACRYLATE • DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER • PEG-10 DIMETHICONE • DISTEARDIMONIUM HECTORITE • TRIMETHYLSILOXYSILICATE • PEG/PPG-36/41 DIMETHYL ETHER • LAVANDULA ANGUSTIFOLIA (LAVENDER) OIL • ALUMINUM DISTEARATE • ALUMINUM HYDROXIDE • ISOSTEARIC ACID • TRISODIUM EDTA • DIPENTAERYTHRITYL HEXAHYDROXYSTEARATE • POLYSILICONE-2 • BHT • TOCOPHEROL • METHICONE • TETRADECENE • PHENOXYETHANOL • IRON OXIDES • MICA • TITANIUM DIOXIDE