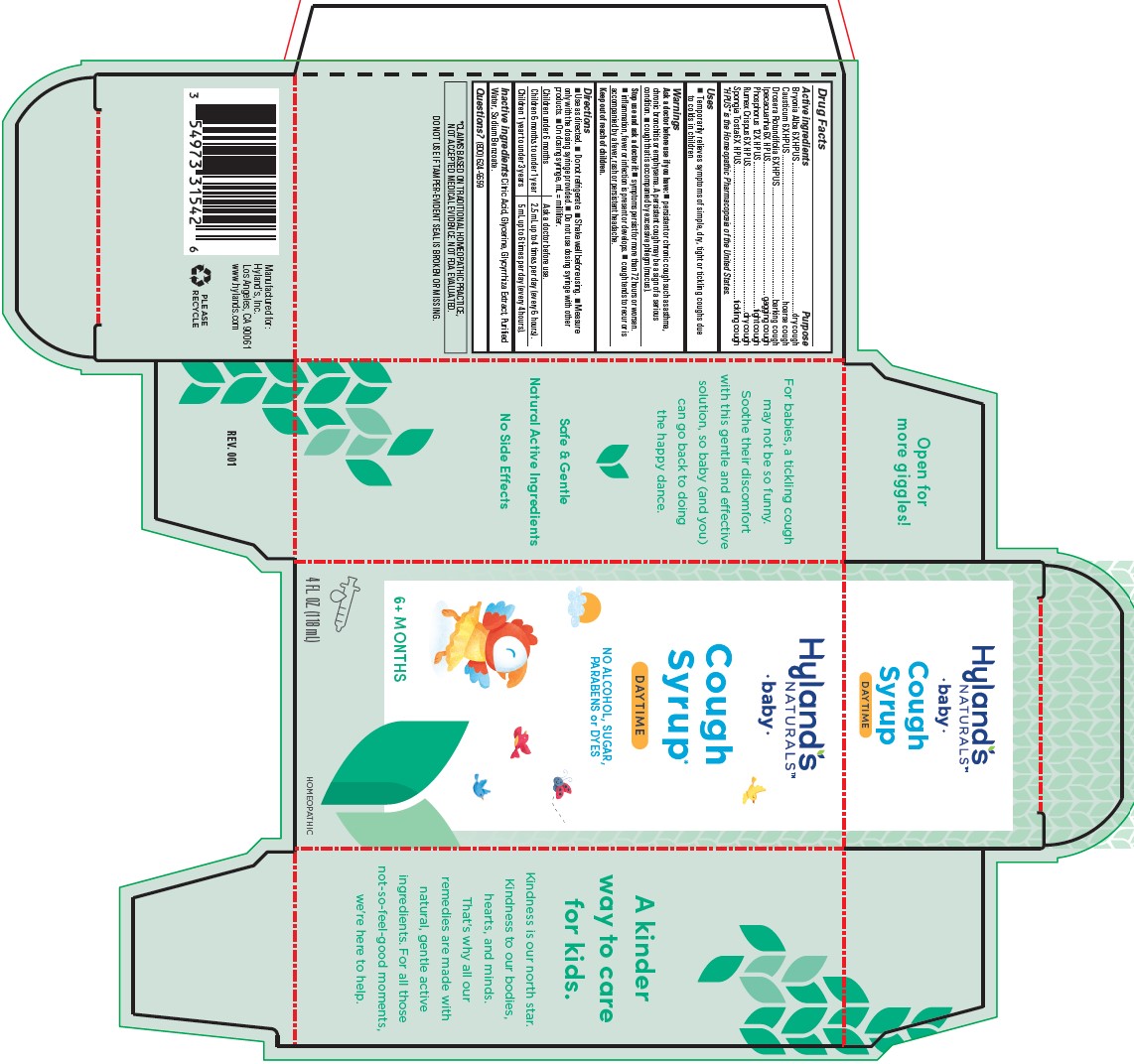
Drug Facts
|
Active ingredients |
Purpose |
|
Bryonia Alba 6X HPUS |
dry cough |
|
Causticum 6X HPUS |
hoarse cough |
|
Drosera Rotundifolia 6X HPUS |
barking cough |
|
Ipecacuanha 6X |
gagging cough |
|
Phosphorus 12X HPUS |
tight cough |
|
Rumex Crispus 6X HPUS |
dry cough |
|
Spongia Tosta 6X HPUS |
tickling cough |
"HPUS" indicates that the active ingredients are in the official Homeopathic
Pharmacopoeia of the United States.
Uses
■ Temporarily relieves the symptoms of simple, dry, tight or tickling coughs due to colds in children.
Warnings
Ask a doctor before use if you have:
■ persistent or chronic cough such as asthma, chronic bronchitis or emphysema. A persistent cough may be a sign of a serious condition. ■ cough that is accompanied by excessive phlegm (mucus).
Directions
■ Use as directed. ■ Do not refrigerate. ■ Shake well before using. Measure
only with the dosing syringe provided. ■ Do not use dosing syringe with other
products. ■ On dosing syringe, mL = milliliter.
| Children under 6 months | Ask a doctor before use. |
| Children 6 months to under 1 year | 2.5 mL up to 4 times per day (every 6 hours). |
| Children 1 year to under 3 years | 5 mL up to 6 times per day (every 4 hours). |