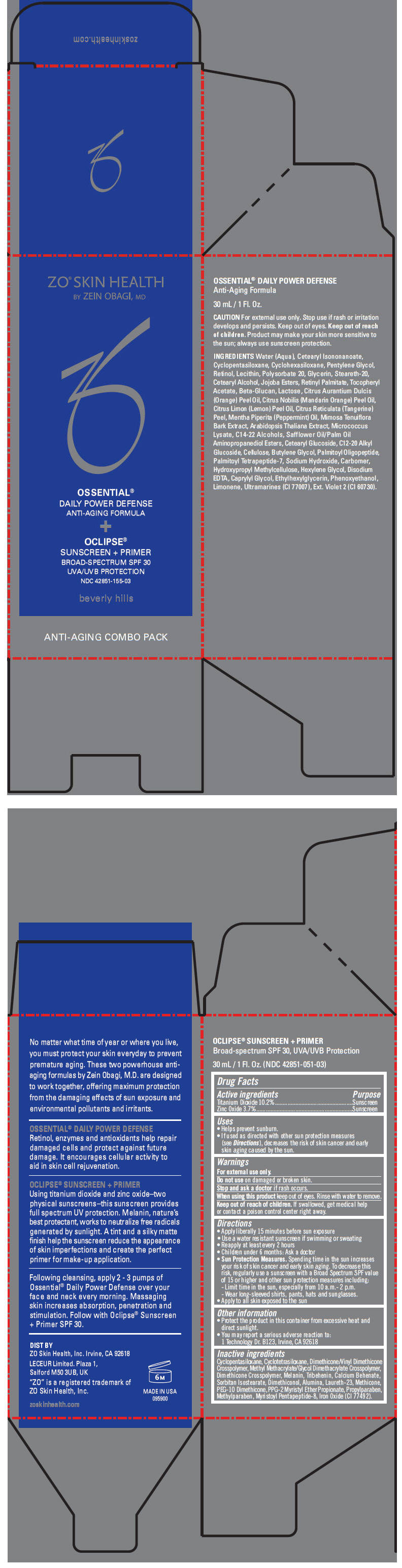
Uses
- Helps prevent sunburn.
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
Directions
- Apply liberally 15 minutes before sun exposure
- Use a water resistant sunscreen if swimming or sweating
- Reapply at least every 2 hours
- Children under 6 months: Ask a doctor
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- -
- Limit time in the sun, especially from 10 a.m.- 2 p.m.
- -
- Wear long-sleeved shirts, pants, hats and sunglasses.
- Apply to all skin exposed to the sun
Other information
- Protect the product in this container from excessive heat and direct sunlight.
- You may report a serious adverse reaction to: 1 Technology Dr. B123, Irvine, CA 92618
Inactive ingredients
Cyclopentasiloxane, Cyclotetrasiloxane, Dimethicone/Vinyl Dimethicone Crosspolymer, Methyl Methacrylate/Glycol Dimethacrylate Crosspolymer, Dimethicone Crosspolymer, Melanin, Tribehenin, Calcium Behenate, Sorbitan Isostearate, Dimethiconol, Alumina, Laureth-23, Methicone, PEG-10 Dimethicone, PPG-2 Myristyl Ether Propionate, Propylparaben, Methylparaben, Myristoyl Pentapeptide-8, Iron Oxide (CI 77492).