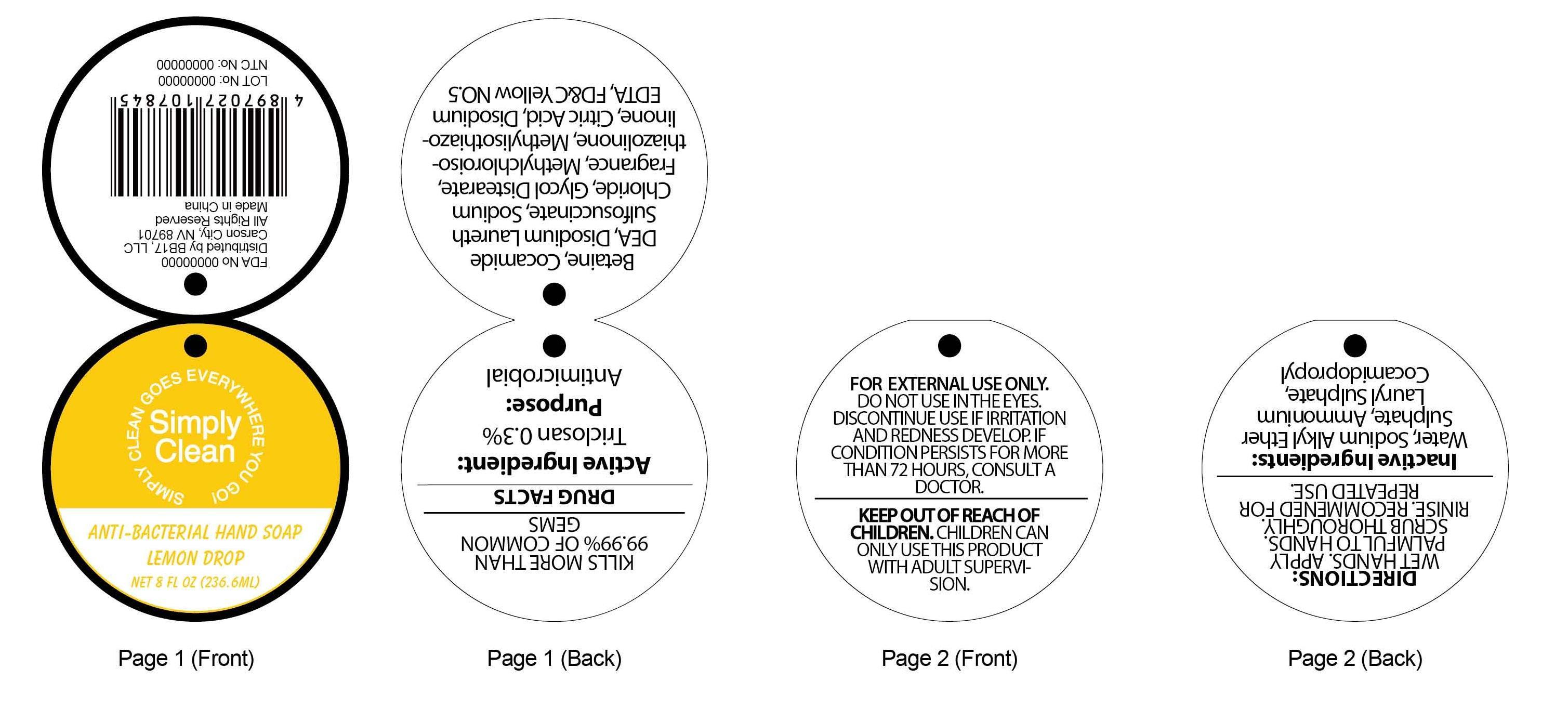
ANTI-BACTERIAL LEMON DROP HAND - triclosan soap
BB17, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient:
Triclosan 0.3%
KILLS MORE THAN 99.99% OF COMMON GERMS
FOR EXTERNAL USE ONLY. DO NOT USE IN THE EYES.
DISCONTINUE USE IF IRRITATION AND REDNESS DEVELOP. IF CONDITION PERSISTS FOR MORE THAN 72 HOURS, CONSULT A DOCTOR.
Keep out of reach of children. CHILDREN CAN ONLY USE THIS PRODUCT WITH ADULT SUPERVISION.
DIRECTIONS:
WET HANDS.APPLY PALMFUL TO HANDS. SCRUB THOROUGHLY. RINISE. RECOMMENDED FOR REPEATED USE.
INACTIVE INGREDIENTS:Water, Sodium Alkyl Ether Sulphate, Ammonium Lauryl Sulphate, Cocamidopropyl Betaine, Cocamide DEA, Disodium Laureth Sulfosuccinate, Sodium Chloride, Glycol Distearate, Fragrance, Methylchloroisothiazolinone, Methylisothiazolinone, Citric Acid, Disodium EDTA, FD&C Yellow NO. 5